4,4-二苯氨基脲 | 603-51-0
中文名称
4,4-二苯氨基脲
中文别名
4,4-二苯基氨脲
英文名称
N,N-diphenyl semicarbazide
英文别名
4,4-diphenylsemicarbazide;4,4-diphenyl semicarbazide;4,4-Diphenyl-semicarbazid;N,N-diphenyl-hydrazinecarboxamide;4,4-Diphenyl-semicarbazol;4,4-Diphenylsemicarbazid;3-amino-1,1-diphenylurea
CAS
603-51-0
化学式
C13H13N3O
mdl
MFCD00007592
分子量
227.266
InChiKey
VVVFQQJJJRFDTE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:151-152 °C(lit.)
-
沸点:368.98°C (rough estimate)
-
密度:1.1267 (rough estimate)
-
稳定性/保质期:
在常温常压下,该物质是稳定的。
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:17
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:58.4
-
氢给体数:2
-
氢受体数:2
安全信息
-
安全说明:S24/25
-
海关编码:2928000090
-
储存条件:本品应密封避光保存。
SDS
| Name: | 4 4-Diphenylsemicarbazide 98% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 603-51-0 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 603-51-0 | 4,4-Diphenylsemicarbazide | 98 | 210-046-5 |
Risk Phrases: 22
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful if swallowed.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
Dust may cause mechanical irritation.
Skin:
No information regarding skin irritation and other potential effects was found.
Ingestion:
The toxicological properties of this substance have not been fully investigated.
Inhalation:
Inhalation of dust may cause respiratory tract irritation.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 603-51-0: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 151.00 - 152.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C13H13N3O
Molecular Weight: 227.27
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 603-51-0 unlisted.
LD50/LC50:
Not available.
Oral, rat: LD50 = 800 Carcinogenicity:
4,4-Diphenylsemicarbazide - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 22 Harmful if swallowed.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 603-51-0: No information available.
Canada
CAS# 603-51-0 is listed on Canada's NDSL List.
CAS# 603-51-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 603-51-0 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
制备方法:能还原斐林氏(Fehling’s)溶液,进行羰基化合物的特性鉴定。有机合成。
用途简介: 暂无内容
用途:能还原斐林氏(Fehling’s)溶液,鉴定羰基化合物的特性。用于有机合成。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,1-二苯基脲 N,N-diphenylurea 603-54-3 C13H12N2O 212.251 二苯氨基甲酰氯 diphenylcarbamic chloride 83-01-2 C13H10ClNO 231.681 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-diphenylcarbamoyl thiosemicarbazide 100871-38-3 C14H14N4OS 286.357 —— diphenylcarbamoyl azide 17223-83-5 C13H10N4O 238.249 —— 1,1-diphenyl-3-n-butylurea 6123-85-9 C17H20N2O 268.359 —— N'-pentyl-N,N-diphenyl-urea 101785-10-8 C18H22N2O 282.385 —— salicylaldehyde-N,N-diphenyl semicarbazone 29416-53-3 C20H17N3O2 331.374
反应信息
-
作为反应物:描述:参考文献:名称:水杨醛半咔唑nes三羰基hen(I)配合物的合成,晶体结构和细胞毒性摘要:合成并表征了一系列N,N-二取代水杨醛半咔唑(SSC)HOC 6 H 4 CH N-NHCONR 2及其their(I)三羰基配合物[ReBr(CO)3(SSC)]。红外和1 H NMR光谱。配合物[ReBr(CO)3(H 2 Bu 2)](H 2 Bu 2 = SSC,其中R = Bu n)的晶体学分析表明,SSC通过其亚氨基氮和羰基氧原子充当二齿配体。[ReBr(CO)3(SSC)]复合物对MOLT-4细胞具有中等至高的细胞毒性(IC 50 = 1–24μM,顺铂为18μM),并且大多数对非癌性人类成纤维细胞无毒。[ReBr(CO)3(H 2 Bnz 2)](Bnz =苄基)的凋亡分析表明,它通过凋亡介导MOLT-4细胞的细胞毒性。配合物[ReBr(CO)3(H 2 Bnz 2)]通过从苯酚OH基团转移到鸟嘌呤N(7)的质子与鸟嘌呤反应。在(CD 3)2 SO中,[ReBr(CO)3(HDOI:10.1016/j.jinorgbio.2012.10.011
-
作为产物:参考文献:名称:Art As Religious Commitment: Kafka’s Debt to Kierkegaardian Ideas and their Impact on his Late Stories摘要:Although Kafka’s reception of Kierkegaardian ideas has received much critical attention the critics have so far paid little heed to similarities between Kierke‐gaard’s religious and Kafka’s aesthetic views. My intention in the following is to show that in spite of Kafka’s critical remarks on his philosophy, Kierkegaard’s definition of a religious person influenced his description of the artist’s existence in Erstes Leid (1922), Ein Hungerkünstler (1922) and Josefine, die Sängerin oder das Volk der Mäuse (1924). In these stories Kafka turns Kierkegaard’s ideas about spiritual inwardness and passionate attitude towards religious life into artistic inwardness and passionate attitude towards art. He also describes how devotion that these artists feel towards their art leads to their solitude and how their lives reflect suffering, doubt and despair which is similar to Kierkegaard’s description of religious suffering. Kafka’s critical remarks on Kierkegaard’s philosophy should therefore be understood as a clear rejection of Kierkegaard’s Protestant theology, although these same ideas gave him inspiration to formulate his views on the artist’s existence.DOI:10.1111/1468-0483.00182
文献信息
-
RECORDING MATERIAL PRODUCED USING NON-PHENOL COMPOUND申请人:NIPPON SODA CO., LTD.公开号:US20150284321A1公开(公告)日:2015-10-08An object of the present invention is to provide a recording material or a recording sheet using, as a color-developing agent, a non-phenol compound that is a safe compound in no danger of corresponding to an endocrine disruptor and is good in color developing performance. The non-phenol compound used in the present invention is at least one selected from the group consisting of compounds represented by the following formulas (I) to (III).
-
Facile one-pot synthesis of 4-substituted semicarbazides作者:Andrey V. Bogolubsky、Yurii S. Moroz、Pavel K. Mykhailiuk、Yurii V. Dmytriv、Sergey E. Pipko、Liudmyla N. Babichenko、Anzhelika I. Konovets、Andrey TolmachevDOI:10.1039/c4ra12425a日期:——and disubstituted semicarbazides was prepared in a one-pot two-step approach. The method includes formation of a carbamate from bis(2,2,2-trifluoroethyl)carbonate or 2,2,2-trifluoroethylchloroformate and a primary or secondary amine and subsequent interaction of the carbamate with hydrazine to result in a semicarbazide. The approach allowed to obtain 4-substituted semicarbazides on a large scale in
-
[EN] DIARYLALKYL CYCLIC DIAMINE DERIVATIVES AS CHEMOKINE RECEPTOR ANTAGONISTS<br/>[FR] DERIVES DE DIAMINE CYCLIQUE DE DIARYLAKLYLE UTILISES EN TANT QU'ANTAGONISTES DES RECEPTEURS DE CHIMIOKINES申请人:TEIJIN LIMITED公开号:WO1997044329A1公开(公告)日:1997-11-27(EN) Cyclic diamines of formula (I) or their pharmacologically acceptable acid addition salts, and their medical applications are described. These compounds inhibit the action of chemokines such as MIP-la and/or MCP-l on target cells, and are useful as a therapeutic drug and/or preventative drug in diseases, such as atherosclerosis, rheumatoid arthritis, and the like where blood monocytes and lymphocytes infiltrate into tissues.(FR) Diamines cycliques de formule (I) ou leur sels d'addition d'acide pharmaceutiquement acceptables ainsi que leurs applications médicales. Ces composés inhibent l'action de chimiokines telles que MIP-1a et/ou MCP-1 sur des cellules cibles, et sont utiles en tant que médicaments thérapeutiques et/ou préventifs dans les maladies telles que l'athérosclérose, l'arthrite rhumatoïde et similaire dans lesquelles les monocytes et les lymphocytes s'infiltrent dans les tissus.
-
Fused benzeneoxyacetic acid derivatives申请人:Ono Pharmaceutical Co., Ltd.公开号:US05596009A1公开(公告)日:1997-01-21A fused benzeneoxyacetic acid derivative of the formula (I): ##STR1## A is --COW, --NR.sup.4 --Y or --Z--NR.sup.4 --CONR.sup.2 R.sup.3 ; and salts thereof possess an agonistic on PGI.sub.2 receptor, so it is useful for prevention and/or treatment of thrombosis, arteriosclerosis, ischemic heart diseases, gastric ulcer and hypertention.
-
Fused benzeneoxyacetic acid derivatives as PGl2 receptor agonists申请人:ONO PHARMACEUTICAL CO., LTD.公开号:EP0542203A2公开(公告)日:1993-05-19A fused benzeneoxyacetic acid derivative of the formula (I): (wherein or A is -COW, -NR4-Y or -Z-NR4 CONR2R3; W is -NR2R3, -NR4-OR5, -NR4-NR2R3 or -NR4-N=CR2R3; Y is -CO-R5, -CO-U-NR2R3 or -CS-U-NR2R3; Z is -CH=N- or -CH2-NR6-; R1 is H or alkyl; R2 or R3 is H, (benzoyl)phenyl or R5; R5 is phenyl, hetero ring containing N atom or alkyl substituted by hetero ring containing one N atom or phenyl; R4 or R6 is H, alkyl or (substituent)phenyl; U is bond or alkylene; e is 3-5; f is 1-3; p or r is 0-4; q is 0-2; s is 0-3) and salts thereof possess an agonistic on PGl2 receptor, so it is useful for prevention and/or treatment of thrombosis, arteriosclerosis, ischemic heart diseases, gastric ulcer and hypertention.式 (I) 的融合苯氧乙酸衍生物: 其中 或 A 是-COW、-NR4-Y 或-Z-NR4 CONR2R3; W 是-NR2R3、-NR4-OR5、-NR4-NR2R3 或 -NR4-N=CR2R3 Y 是-CO-R5、-CO-U-NR2R3 或-CS-U-NR2R3; Z是-CH=N-或-CH2-NR6-; R1 是 H 或烷基; R2 或 R3 是 H、(苯甲酰基)苯基或 R5; R5 是苯基、含 N 原子的杂环或被含一个 N 原子的杂环或苯基取代的烷基; R4 或 R6 是 H、烷基或(取代基)苯基; U 是键或亚烷基; e 是 3-5; f 为 1-3; p 或 r 是 0-4; q 为 0-2; s 为 0-3) 及其盐类对 PGl2 受体具有激动作用,因此可用于预防和/或治疗血栓形成、动脉硬化、缺血性心脏病、胃溃疡和高血压。
表征谱图
-
氢谱1HNMR
-
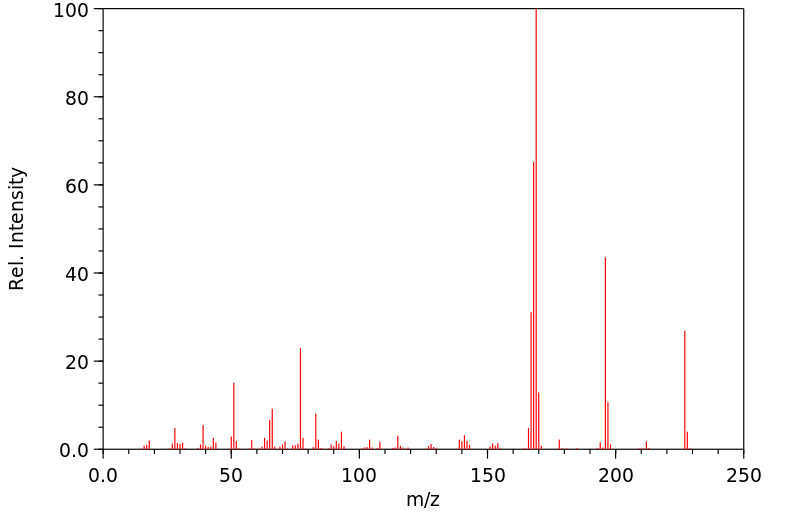
质谱MS
-
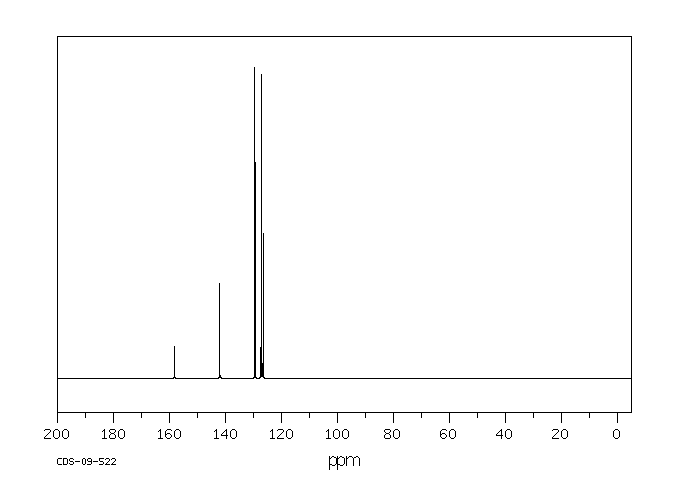
碳谱13CNMR
-
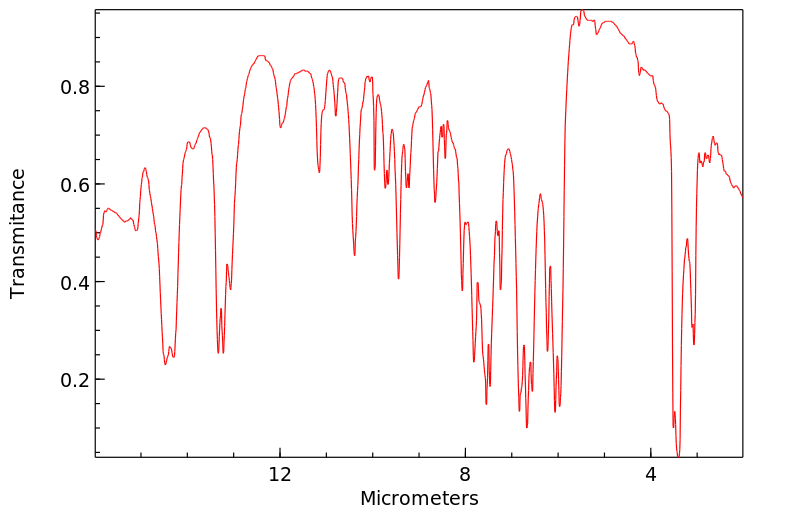
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫