4-(2,3,6-三甲基苯基)-3-丁烯-2-酮 | 56681-06-2
中文名称
4-(2,3,6-三甲基苯基)-3-丁烯-2-酮
中文别名
——
英文名称
4--buten-(3)-on-(2)
英文别名
4-<2',3',6'-Trimethyl-phenyl>-buten-(3)-on-(2);4-(2,3,6-Trimethylphenyl)-3-buten-2-on;4-(2,3,4-Trimethylphenyl)-3-buten-2-on;4-<2,3,6-Trimethyl-phenyl-(1)>-buten-(3)-on-(2);4-(2,3,6-Trimethylphenyl)but-3-en-2-one
CAS
56681-06-2
化学式
C13H16O
mdl
——
分子量
188.269
InChiKey
AHHGVKNOSDJAQN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
LogP:3.550 (est)
-
稳定性/保质期:
存在于烤烟烟叶、白肋烟烟叶以及烟气中。
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:14
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.31
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
制备方法与用途
制备方法
- 烟草: BU,14; FC,9。
- 烟草:BU,14;FC,9。
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— isorenieratene 524-01-6 C40H48 528.821
反应信息
-
作为反应物:描述:参考文献:名称:异肾上腺素XXIX的合成。关于类胡萝卜素合成的交流摘要:合成地生产了在橙色海绵日本海Ren中形成的多烯染料异雷尼丁,因此证实了由MASARU YAMAGUCHI建立的结构式。DOI:10.1002/hlca.19600430158
-
作为产物:描述:参考文献:名称:异肾上腺素XXIX的合成。关于类胡萝卜素合成的交流摘要:合成地生产了在橙色海绵日本海Ren中形成的多烯染料异雷尼丁,因此证实了由MASARU YAMAGUCHI建立的结构式。DOI:10.1002/hlca.19600430158
文献信息
-
A further step to sustainable palladium catalyzed oxidation: Allylic oxidation of alkenes in green solvents作者:Maíra dos Santos Costa、Amanda de Camargo Faria、Rayssa L.V. Mota、Elena V. GusevskayaDOI:10.1016/j.apcata.2021.118349日期:2021.9The palladium catalyzed oxidation of alkenes with molecular oxygen is a synthetically important reaction which employs palladium catalysts in solution; therefore, a solvent plays a critical role for the process. In this study, we have tested several green solvents as a reaction medium for the allylic oxidation of a series of alkenes. Dimethylcarbonate, methyl isobutyl ketone, and propylene carbonate
-
Total Synthesis of Natural Acetylenic Analogues of Isorenieratene and Renieratene作者:Tetsuji Ike、Junji Inanaga、Akio Nakano、Nobuhisa Okukado、Masaru YamaguchiDOI:10.1246/bcsj.47.350日期:1974.2two natural acetylenic aromatic carotenoids, 7,8-didehydroisorenieratene (1) and 7,8-didehydrorenieratene (2), was carried out. Although the condensations involving C15-acetylenic ylids (derived from the phosphonium salts 7, and 8) as the intermediates gave only 9-cis isomers of 1 and 2, low-temperature condensations of C25-hexaene yield (derived from 19 and 28) with C15-acetylenic aldehyde (12a) led
表征谱图
-
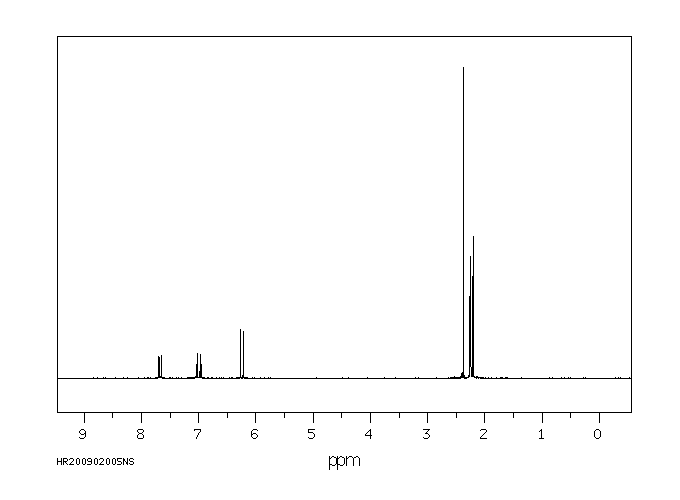
氢谱1HNMR
-
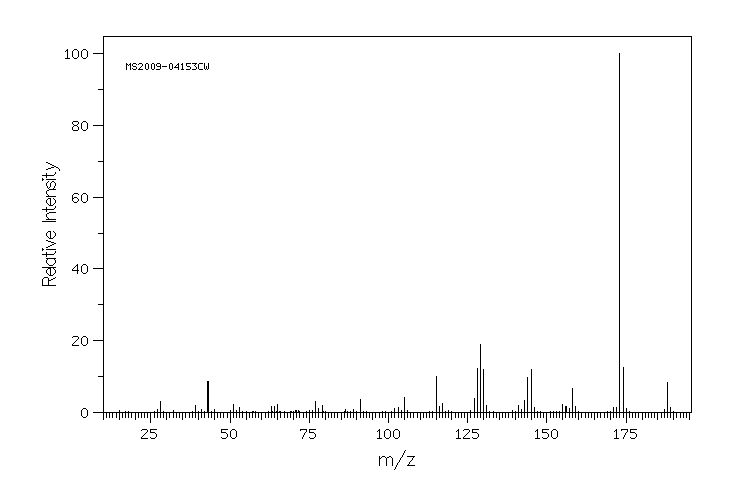
质谱MS
-
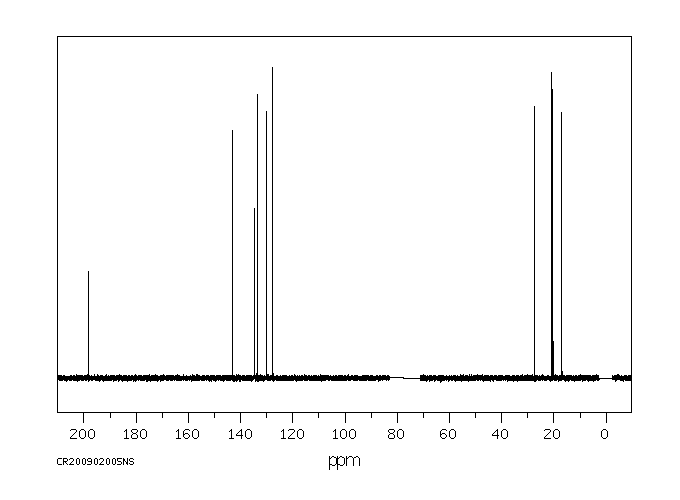
碳谱13CNMR
-
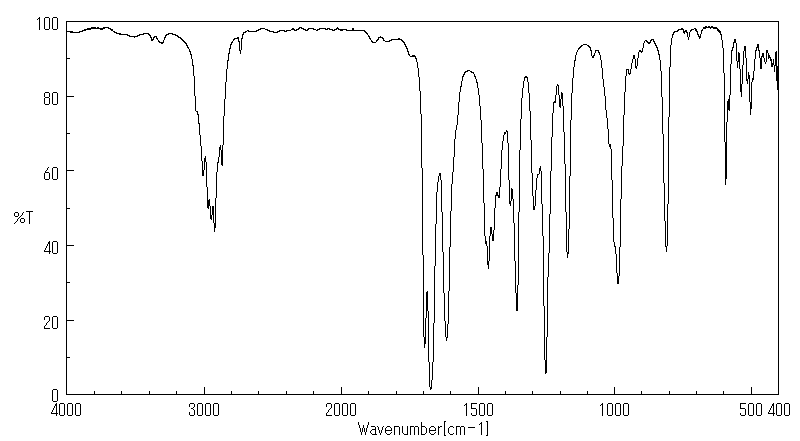
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(E)-3-(4-(叔丁基)苯基)丙烯酸乙酯
(E)-3-(2-(三氟甲基)苯基)丙烯酸乙酯
(E)-3-(2,4-二甲氧基苯基)丙烯酸乙酯
(2E)-N-[2-(3-羟基-2-氧代-2,3-二氢-1H-吲哚-3-基)乙基]-3-苯基丙-2-烯酰胺
黄金树苷
鲁索曲波帕
香豆酸肉桂酯
香豆酰多巴胺
香草醛缩丙酮
顺式邻羟基肉桂酸
顺式芥子酸
顺式-曲尼司特
顺式-乙基肉桂酸酯
顺式-N-阿魏酰酪胺
顺式-3,4-二甲氧基苯丙烯酸
顺式-2-((叔丁氧羰基)氨基)-3-(4-氨甲酰基-2,6-二甲苯基)丙烯酸甲酯
顺-o-羧基肉桂酸
顺-2-甲氧基肉桂酸
阿魏酸钠
阿魏酸酰胺
阿魏酸甲酯
阿魏酸甲酯
阿魏酸甲酯
阿魏酸松柏酯
阿魏酸杂质1
阿魏酸异辛酯
阿魏酸哌嗪
阿魏酸二十烷基酯
阿魏酸乙酯
阿魏酸4-O-硫酸二钠盐
阿魏酸-D3
阿魏酸
阿魏酸
阿魏酰酪胺
间羟基肉桂酸
间羟基肉桂酸
间硝基肉桂酸
间甲基肉桂酸
间甲基反式肉桂酸甲酯
间氯肉桂酸
间三氟甲氧基肉桂酸甲酯
间-香豆酸
间-(三氟甲基)-肉桂酸
锂(E)-2-溴-3-苯基丙烯酸酯
钠二乙基2-[(氧代氨基)-苯基亚甲基]丙二酸酯盐
酪氨酸磷酸化抑制剂AG 556
酪氨酸磷酸化抑制剂AG 527
酪氨酸磷酸化抑制剂AG 490
酪氨酸磷酸化抑制剂A46
酪氨酸磷酸化抑制剂 AG 30