4-氯-6-乙基氨基嘧啶 | 872511-30-3
中文名称
4-氯-6-乙基氨基嘧啶
中文别名
6-氯-N-乙基嘧啶-4-胺
英文名称
(6-chloro-pyrimidin-4-yl)ethylamine
英文别名
6-chloro-N-ethylpyrimidin-4-amine;4-chloro-6-ethylaminopyrimidine;4-ethylamino-6-chloropyrimidine;(6-chloro-pyrimidin-4-yl)-ethyl-amine
CAS
872511-30-3
化学式
C6H8ClN3
mdl
MFCD09475892
分子量
157.603
InChiKey
PCOIHHDNCUROLX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:290℃
-
密度:1.273
-
闪点:129℃
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:10
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:37.8
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
危险类别码:R22
-
海关编码:2933599090
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 4-Chloro-6-ethylaminopyrimidine
Synonyms: (6-Chloropyrimidin-4-yl)ethylamine; 6-Chloro-N-ethylpyrimidin-4-amine
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 4-Chloro-6-ethylaminopyrimidine
CAS number: 872511-30-3
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C6H8ClN3
Molecular weight: 157.6
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen chloride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 4-Chloro-6-ethylaminopyrimidine
Synonyms: (6-Chloropyrimidin-4-yl)ethylamine; 6-Chloro-N-ethylpyrimidin-4-amine
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 4-Chloro-6-ethylaminopyrimidine
CAS number: 872511-30-3
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C6H8ClN3
Molecular weight: 157.6
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen chloride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:4-氯-6-乙基氨基嘧啶 在 盐酸 作用下, 以 1,4-二氧六环 、 甲苯 、 正丁醇 为溶剂, 反应 48.0h, 生成 3-(2-chloro-3,5-dimethoxyphenyl)-1-ethyl-1-{6-[4-(4-methylpiperazin-1-yl)phenylamino]pyrimidin-4-yl}urea参考文献:名称:Discovery of 3-(2,6-Dichloro-3,5-dimethoxy-phenyl)-1-{6-[4-(4-ethyl-piperazin-1-yl)-phenylamino]-pyrimidin-4-yl}-1-methyl-urea (NVP-BGJ398), A Potent and Selective Inhibitor of the Fibroblast Growth Factor Receptor Family of Receptor Tyrosine Kinase摘要:A novel series of N-aryl-N'-pyrimidin-4-yl ureas has been optimized to afford potent and selective inhibitors of the fibroblast growth factor receptor tyrosine kinases 1, 2, and 3 by rationally designing the substitution pattern of the aryl ring. On the basis of its in vitro profile, compound 1h (NVP-BGJ398) was selected for in vivo evaluation and showed significant antitumor activity in RT112 bladder cancer xenografts models overexpressing wild-type FGFR3. These results support the potential therapeutic use of 1h as a new anticancer agent.DOI:10.1021/jm2006222
-
作为产物:参考文献:名称:Compounds and Compositions as Protein Kinase Inhibitors摘要:本发明涉及公式(I)化合物,其中取代基X1,R1,R2,R3和R4的含义如本发明说明书所述和解释的那样,以及制备这些化合物的过程,包含它们的药物组合物,可选择与一种或多种其他药物活性化合物联合使用以治疗对蛋白激酶活性抑制有反应的疾病,以及治疗这种疾病的方法。公开号:US20090137804A1
文献信息
-
具有Akt抑制活性的取代嘧啶类衍生物及其制 备方法与应用
-
Chemoselective Reactions of 4,6-Dichloro-2-(methylsulfonyl)pyrimidine and Related Electrophiles with Amines作者:Young-Choon Moon、Ramil Baiazitov、Wu Du、Chang-Sun Lee、Seongwoo Hwang、Neil AlmsteadDOI:10.1055/s-0033-1338853日期:——Chemoselective SNAr reactions of 4,6-dichloro-2-(methylsulfonyl)pyrimidine and several related electrophiles with amines and their derivatives are described. In the presence of weak bases anilines and secondary aliphatic amines selectively displace the chloride group. Deprotonated anilines and their carbonyl derivatives displace the sulfone group. Sterically and electronically unbiased primary aliphatic amines献给斯科特E.丹麦教授在他的60之际个生日 抽象 描述了4,6-二氯-2-(甲基磺酰基)嘧啶和几种相关亲电试剂与胺及其衍生物的化学选择性S NAr反应。在弱碱的存在下,苯胺和脂肪族仲胺选择性取代氯基。去质子化的苯胺及其羰基衍生物取代了砜基。立体和电子无偏伯脂肪族胺选择性取代4,6-二氯-2-(甲基磺酰基)嘧啶中的砜基;然而,它们与其他亲电试剂的反应通常选择性较低。提出了立体驱动的选择性解释。 描述了4,6-二氯-2-(甲基磺酰基)嘧啶和几种相关亲电试剂与胺及其衍生物的化学选择性S NAr反应。在弱碱的存在下,苯胺和脂肪族仲胺选择性取代氯基。去质子化的苯胺及其羰基衍生物取代了砜基。立体和电子无偏伯脂肪族胺选择性取代4,6-二氯-2-(甲基磺酰基)嘧啶中的砜基;然而,它们与其他亲电试剂的反应通常选择性较低。提出了立体驱动的选择性解释。
-
[EN] FGFR4 INHIBITORS<br/>[FR] INHIBITEURS DE FGFR4申请人:EISAI R&D MAN CO LTD公开号:WO2016164754A1公开(公告)日:2016-10-13We provide FGFR inhibitors, their salts, methods of manufacture, and methods of use.我们提供FGFR抑制剂,它们的盐,制造方法和使用方法。
-
[EN] ANTIVIRAL COMPOUNDS<br/>[FR] COMPOSÉS ANTIVIRAUX申请人:UNIV WIEN公开号:WO2020221894A1公开(公告)日:2020-11-05The invention is provides novel antiviral compounds, as well as derivatives thereof. The compounds of the invention are preferably formulated as pharmaceuticals. The invention provides the compounds for use in the prevention and treatment of infectious diseases, in particular viral diseases. In some aspects the invention is based on the antiviral activity of the provided compounds against the Chikungunya virus, and hence, their application in the treatment or prevention of any physiological manifestation of such viral infection.这项发明提供了新型抗病毒化合物,以及其衍生物。该发明的化合物通常被制成药物。该发明提供了这些化合物用于预防和治疗传染病,特别是病毒性疾病。在某些方面,该发明基于所提供的化合物对基孔肯亚病毒的抗病毒活性,因此,它们在治疗或预防此类病毒感染的任何生理表现中的应用。
-
[EN] NEW TRPA1 ANTAGONISTS<br/>[FR] NOUVEAUX ANTAGONISTES DE TRPA1申请人:ALMIRALL SA公开号:WO2017060488A1公开(公告)日:2017-04-13The present invention relates to compounds of Formula (I), to the process for preparing such compounds and to their use in the treatment of a pathological condition or disease susceptible to amelioration by TRPA1 channel inhibition or antagonism.本发明涉及式(I)的化合物,以及制备这种化合物的方法,以及它们在治疗通过TRPA1通道抑制或拮抗可改善的病理状况或疾病中的用途。
表征谱图
-
氢谱1HNMR
-
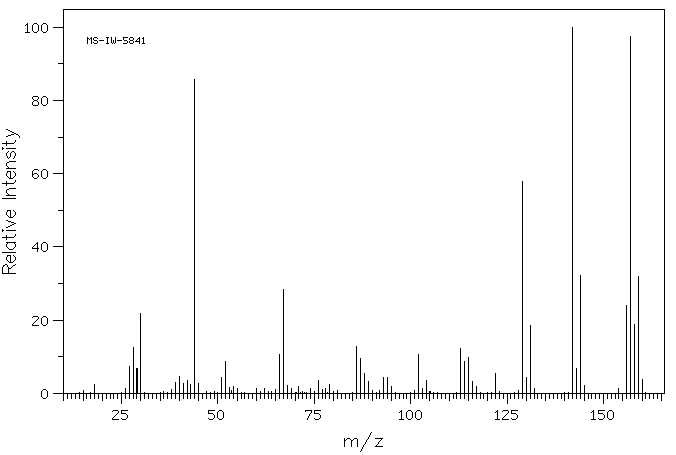
质谱MS
-
碳谱13CNMR
-
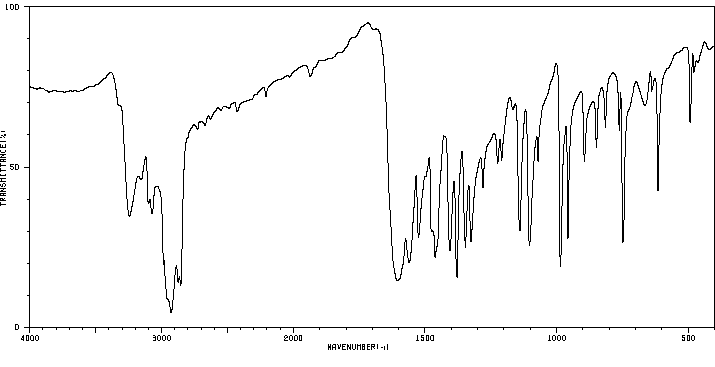
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-3-(2-(二氟甲基)吡啶-4-基)-7-氟-3-(3-(嘧啶-5-基)苯基)-3H-异吲哚-1-胺
(6-羟基嘧啶-4-基)乙酸
(4,5-二甲氧基-1,2,3,6-四氢哒嗪)
鲁匹替丁
马西替坦杂质7
马西替坦杂质4
马西替坦杂质
马西替坦原料药杂质D
马西替坦原料药杂质B
马西替坦
顺式-4-{[5-溴-2-(2,5-二甲基-1H-吡咯-1-基)-6-甲基嘧啶-4-基]氨基}环己醇
非沙比妥
非巴氨酯
非尼啶醇
青鲜素钾盐
雷特格韦钾盐
雷特格韦相关化合物E(USP)
雷特格韦杂质8
雷特格韦EP杂质H
雷特格韦-RT9
雷特格韦
阿西莫司杂质3
阿西莫司
阿脲四水合物
阿脲一水合物
阿维霉素
阿米美啶
阿米洛利
阿米妥钠
阿洛巴比妥
阿普瑞西他滨
阿普比妥
阿巴卡韦相关化合物B(USP)
阿卡明
阿伐那非杂质V
阿伐那非杂质1
阿伐那非杂质
阿伐那非中间体
阿伐那非
铂(2+)二氯化6-甲基-1,3-二{2-[(2-甲基丙基)硫烷基]乙基}嘧啶-2,4(1H,3H)-二酮(1:1)
钴1,2,3,6-四氢-2,6-二氧代嘧啶-4-羧酸酯(1:2)
钠5-烯丙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-乙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-(2-溴丙-2-烯基)-5-丁烷-2-基-4,6-二氧代-1H-嘧啶-2-醇
醌肟腙
酒石酸噻吩嘧啶
那可比妥
辛基2,6-二氧代-1,2,3,6-四氢-4-嘧啶羧酸酯
赛乐西帕杂质3
赛乐西帕KSM3