绿草定 | 55335-06-3
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:148-150°C
-
沸点:290°C
-
密度:1.7626 (rough estimate)
-
溶解度:可溶于氯仿(少许)、乙酸乙酯(少许)、甲醇(少许)
-
物理描述:WHITE OR COLOURLESS FLUFFY CRYSTALS.
-
颜色/状态:Fluffy solid
-
蒸汽压力:1.26X10-6 mm Hg at 25 °C
-
稳定性/保质期:
如果按照规格正确使用和储存,则不会发生分解,且没有已知的危险反应。
对兔眼睛有轻微刺激作用,但对皮肤无刺激性。在实验条件下,未观察到动物出现致畸、致癌或致突变现象。
-
分解:Decomposes at 208 °C.
-
解离常数:pKa = 2.68
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:14
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:59.4
-
氢给体数:1
-
氢受体数:4
ADMET
安全信息
-
危险品标志:Xn
-
安全说明:S22,S24/25
-
危险类别码:R22
-
WGK Germany:3
-
危险品运输编号:61894
-
RTECS号:AJ9000000
-
海关编码:3808931100
-
储存条件:密封,在0至6℃下保存
SDS
| 第一部分:化学品名称 |
| 化学品中文名称: | 绿草定;(3,5,6-三氯-2-吡啶基)氧乙酸 |
| 化学品英文名称: | Triclopyr;[(3,5,6-Trichloro-2-pyridyl)oxy]aceticacid |
| 中文俗名或商品名: | |
| Synonyms: | |
| CAS No.: | 55335-06-3 |
| 分子式: | C 7 H 4 Cl 3 NO 3 |
| 分子量: | 256.47 |
| 第二部分:成分/组成信息 |
| 纯化学品 混合物 | |||
| 化学品名称:绿草定;(3,5,6-三氯-2-吡啶基)氧乙酸 | |||
|
| 第三部分:危险性概述 |
| 危险性类别: | 第6.1类 毒害品 |
| 侵入途径: | 吸入 食入 经皮吸收 |
| 健康危害: | 本品为低毒除草剂。误服有毒。对眼睛有刺激作用。有致畸作用。受热分解释出氯和氮氧化物。 |
| 环境危害: | |
| 燃爆危险: |
| 第四部分:急救措施 |
| 皮肤接触: | 用肥皂水及清水彻底冲洗。就医。 |
| 眼睛接触: | 拉开眼睑,用流动清水冲洗15分钟。就医。 |
| 吸入: | 脱离现场至空气新鲜处。就医。 |
| 食入: | 误服者,饮适量温水,催吐。就医。 |
| 第五部分:消防措施 |
| 危险特性: | 遇明火、高热可燃。受高热分解,放出有毒的烟气。 |
| 有害燃烧产物: | |
| 灭火方法及灭火剂: | 泡沫、干粉、砂土。 |
| 消防员的个体防护: | |
| 禁止使用的灭火剂: | |
| 闪点(℃): | |
| 自燃温度(℃): | |
| 爆炸下限[%(V/V)]: | |
| 爆炸上限[%(V/V)]: | |
| 最小点火能(mJ): | |
| 爆燃点: | |
| 爆速: | |
| 最大燃爆压力(MPa): | |
| 建规火险分级: |
| 第六部分:泄漏应急处理 |
| 应急处理: | 隔离泄漏污染区,周围设警告标志,建议应急处理人员戴好防毒面具,穿化学防护服。小心扫起,置于袋中转移至安全场所。用水刷洗泄漏污染区,经稀释的污水放入废水系统。如大量泄漏,收集回收或无害处理后废弃。 |
| 第七部分:操作处置与储存 |
| 操作注意事项: | |
| 储存注意事项: |
| 第八部分:接触控制/个体防护 |
| 最高容许浓度: | 中 国 MAC:未制订标准前苏联 MAC:未制订标准美国TLV—TWA:未制订标准 |
| 监测方法: | |
| 工程控制: | 密闭操作,局部排风。 |
| 呼吸系统防护: | 生产操作或农业使用时,应该佩戴防尘口罩。紧急事态抢救或逃生时,佩戴防毒面具。 |
| 眼睛防护: | 戴化学安全防护眼镜。 |
| 身体防护: | 穿相应的防护服。 |
| 手防护: | 戴防护手套。 |
| 其他防护: | 工作现场禁止吸烟、进食和饮水。工作后,淋浴更衣。注意个人清洁卫生。 |
| 第九部分:理化特性 |
| 外观与性状: | 原药为白色固体。 |
| pH: | |
| 熔点(℃): | 148~150 |
| 沸点(℃): | |
| 相对密度(水=1): | |
| 相对蒸气密度(空气=1): | |
| 饱和蒸气压(kPa): | 0.168*(10-6)/25℃ |
| 燃烧热(kJ/mol): | |
| 临界温度(℃): | |
| 临界压力(MPa): | |
| 辛醇/水分配系数的对数值: | |
| 闪点(℃): | |
| 引燃温度(℃): | |
| 爆炸上限%(V/V): | |
| 爆炸下限%(V/V): | |
| 分子式: | C 7 H 4 Cl 3 NO 3 |
| 分子量: | 256.47 |
| 蒸发速率: | |
| 粘性: | |
| 溶解性: | 微溶于水,溶于乙醇、丙酮等。 |
| 主要用途: | 用作农用除草剂。 |
| 第十部分:稳定性和反应活性 |
| 稳定性: | 在常温常压下 稳定 |
| 禁配物: | 强氧化剂。 |
| 避免接触的条件: | |
| 聚合危害: | 不能出现 |
| 分解产物: | 一氧化碳、二氧化碳、氮氧化物、氯化氢。 |
| 第十一部分:毒理学资料 |
| 急性毒性: | LD50:729mg/kg(大鼠经口);350mg/kg(兔经皮) LC50: |
| 急性中毒: | |
| 慢性中毒: | |
| 亚急性和慢性毒性: | |
| 刺激性: | |
| 致敏性: | |
| 致突变性: | |
| 致畸性: | |
| 致癌性: |
| 第十二部分:生态学资料 |
| 生态毒理毒性: | |
| 生物降解性: | |
| 非生物降解性: | |
| 生物富集或生物积累性: |
| 第十三部分:废弃处置 |
| 废弃物性质: | |
| 废弃处置方法: | |
| 废弃注意事项: |
| 第十四部分:运输信息 |
| |
| 危险货物编号: | 61894 |
| UN编号: | 3027 |
| 包装标志: | |
| 包装类别: | |
| 包装方法: | |
| 运输注意事项: | 储存于阴凉、通风仓间内。远离火种、热源。专人保管。保持容器密封防止受潮和雨淋。防止阳光曝晒。不能与粮食、食物、种子、饲料、各种日用品混装混运。操作现场不得吸烟、饮水、进食。搬运时要轻装轻卸,防止包装及 |
| RETCS号: | |
| IMDG规则页码: |
| 第十五部分:法规信息 |
| 国内化学品安全管理法规: | |
| 国际化学品安全管理法规: |
| 第十六部分:其他信息 |
| 参考文献: | 1.周国泰,化学危险品安全技术全书,化学工业出版社,1997 2.国家环保局有毒化学品管理办公室、北京化工研究院合编,化学品毒性法规环境数据手册,中国环境科学出版社.1992 3.Canadian Centre for Occupational Health and Safety,CHEMINFO Database.1998 4.Canadian Centre for Occupational Health and Safety, RTECS Database, 1989 |
| 填表时间: | 年月日 |
| 填表部门: | |
| 数据审核单位: | |
| 修改说明: | |
| 其他信息: | 6 |
| MSDS修改日期: | 年月日 |
制备方法与用途
绿草定的主要成分是三氯吡氧乙酸,该成分目前在国内主要与草甘膦登记为混剂,登记的剂型都是可湿性粉剂,使用范围主要是非耕地防除阔叶杂草和小灌木等。在许多欧美国家,该成分还登记应用在水稻、甘蔗和玉米等禾本科作物上,而国内在这类作物上的应用仍处于空白阶段。
毒性大鼠急性经口LD50为729mg/kg(雄)/630mg/kg(雌),家兔急性经皮LD50为350mg/kg。对兔眼睛有轻度刺激,但对皮肤无刺激作用。大鼠亚急性经口无作用剂量为每天5.5mg/kg,慢性经口无作用剂量为每天30mg/kg。在试验条件下,未见动物致畸、致癌或致突变作用。
化学性质纯品为白色结晶固体,熔点148~150℃,分解温度290℃,蒸气压1.68×10-4Pa。能溶于乙醇等有机溶剂,在25℃时水中溶解度为430~440mg/L。在土壤中的半衰期约为46天。
用途三氯吡氧乙酸作用于核酸代谢,使植物产生过量的核酸,导致叶片、茎和根部畸形,贮藏物质耗尽,维管束组织栓塞或破裂,最终导致植株死亡。该产品适用于造林前除草灭灌、维护防火线以及扶育松树及林分改造等场景,也可防除多种杂草如胡枝子、榛材、蒙古柞等多种植物。
对于幼林抚育的用量为1kg有效成分/hm2;造林前及防火线则需要使用1.95kg有效成分/hm2。值得注意的是,对松树和云杉类植物应严格控制剂量,若超过1kg/hm2可能会产生药害。
此外,该产品还可用于稻田、小麦杂草防治及种植物园(如油棕和橡胶)等。例如,90g(a.i.)/ha与2,4-滴或敌稗混用可防除稻田和小麦田(不适用于大麦田)的杂草;也可用于种植园(如油棕和橡胶),使用125~250g(a.i.)/ha可防除覆盖植物,用0.72~10kg(a.i.)/ha则可防除泽兰属及其他主要杂草。在牧场中,用1~2kg(a.i.)/ha防除一年生和多年生草本杂草;以2~4kg/ha用于悬钩子属和其他木本杂草的防治;在森林处理时,分别使用4~8kg/ha进行生育处理、1.5~2.0kg/ha针叶树处理及2~8kg/ha的工业区除草作业。放牧地除草则需用量为0.24~10kg/ha。
生产方法 类别农药
毒性分级中毒
急性毒性口服 - 大鼠 LD50: 630 毫克/公斤
可燃性危险特性燃烧时产生有毒氮氧化物和氯化物气体
储运特性库房应保持通风、低温干燥,并与食品原料分开储运
灭火剂干粉、泡沫、砂土
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 绿草定甲酯 methyl 2-((3,5,6-trichloropyridin-2-yl)oxy)acetate 60825-26-5 C8H6Cl3NO3 270.5 (3,5,6-三氯-2-吡啶基)氧基-乙酸乙酯 ethyl 2-((3,5,6-trichloropyridin-2-yl)oxy) acetate 60825-27-6 C9H8Cl3NO3 284.526 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 绿草定甲酯 methyl 2-((3,5,6-trichloropyridin-2-yl)oxy)acetate 60825-26-5 C8H6Cl3NO3 270.5 绿草定-2-丁氧基乙酯 butoxyethyl ester of [(3,5,6-trichloro-2-pyridyl)oxy]acetic acid 64700-56-7 C13H16Cl3NO4 356.633 —— triclopyr acetyl chloride 81695-17-2 C7H3Cl4NO2 274.918 —— N-(n-propyl)-3,5,6-trichloropyridyloxyacetamide 99938-54-2 C10H11Cl3N2O2 297.569
反应信息
-
作为反应物:描述:参考文献:名称:Fungicidal N-pyridyloxy alkyl amines摘要:该化合物的结构式为:##STR1## 其中 X 为氧或硫;R 为吡啶基,可选地取代为1至3个卤素、较低的烷基或较低的烷氧基,可选地取代为1至3个相同或不同的卤素原子或硝基;R.sup.1 为较低的烷基;R.sup.2 为含有1至3个环中氮原子和其余环原子为碳原子的5-或6-成员杂环,喹啉环或苯环,均可选地取代为1至3个卤素、三卤甲基、较低的烷基或较低的烷氧基,前提是 R.sup.2 未通过环氮与 --CH.sub.2 -- 基团相连,以及其相容盐,具有杀真菌作用。公开号:US04544661A1
-
作为产物:描述:参考文献:名称:Monitoring of the Organophosphate Pesticide Chlorpyrifos in Vegetable Samples from Local Markets in Northern Thailand by Developed Immunoassay摘要:氯氰菊酯是一种有机磷杀虫剂,广泛用于农民进行作物保护。然而,人们对其在环境和食物链中的污染存在担忧。在本研究中,开发并验证了一种用于检测氯氰菊酯的室内间接竞争酶联免疫吸附测定法(ic-ELISA),并与气相色谱-火焰光度检测(GC-FPD)作为传统方法进行了比较。开发的ic-ELISA被用于检测蔬菜样品中的氯氰菊酯残留。开发的ic-ELISA对氯氰菊酯表现出良好的灵敏度,IC50为0.80 µg/kg,对其他有机磷杀虫剂的交叉反应性低。从泰国北部的清莱、清迈和南府的当地市场收集了160个样品。蔬菜样品中氯氰菊酯残留的阳性率为33.8%,黄瓜、香菜和空心菜中发现的氯氰菊酯水平最高,分别为275、145和35.3 µg/kg。在检测到的样品中,氯氰菊酯的最高中位数水平分别为大白菜(332 μg/kg)、黄瓜(146.3 μg/kg)和芥兰(26.95 μg/kg)。开发的ic-ELISA适用于快速定量氯氰菊酯残留。DOI:10.3390/ijerph17134723
文献信息
-
[EN] ACC INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE L'ACC ET UTILISATIONS ASSOCIÉES
-
[EN] 3-[(HYDRAZONO)METHYL]-N-(TETRAZOL-5-YL)-BENZAMIDE AND 3-[(HYDRAZONO)METHYL]-N-(1,3,4-OXADIAZOL-2-YL)-BENZAMIDE DERIVATIVES AS HERBICIDES<br/>[FR] DÉRIVÉS DE 3-[(HYDRAZONO))MÉTHYL]-N-(TÉTRAZOL-5-YL)-BENZAMIDE ET DE 3-[(HYDRAZONO)MÉTHYL]-N-(1,3,4-OXADIAZOL-2-YL)-BENZAMIDE UTILISÉS EN TANT QU'HERBICIDES申请人:SYNGENTA CROP PROTECTION AG公开号:WO2021013969A1公开(公告)日:2021-01-28The present invention related to compounds of Formula (I): or an agronomically acceptable salt thereof, wherein Q, R2, R3, R4, R5 and R6 are as described herein. The invention further relates to compositions comprising said compounds, to methods of controlling weeds using said compositions, and to the use of compounds of Formula (I) as a herbicide.本发明涉及以下式(I)的化合物或其农业上可接受的盐,其中Q、R2、R3、R4、R5和R6如本文所述。该发明还涉及包含所述化合物的组合物,使用这些组合物控制杂草的方法,以及将式(I)的化合物用作除草剂的用途。
-
[EN] INSECTICIDAL TRIAZINONE DERIVATIVES<br/>[FR] DÉRIVÉS DE TRIAZINONE INSECTICIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2013079350A1公开(公告)日:2013-06-06Compounds of the formula (I) or (I'), wherein the substituents are as defined in claim 1, are useful as pesticides.式(I)或(I')的化合物,其中取代基如权利要求1所定义的那样,可用作杀虫剂。
-
[EN] HERBICIDALLY ACTIVE HETEROARYL-S?BSTIT?TED CYCLIC DIONES OR DERIVATIVES THEREOF<br/>[FR] DIONES CYCLIQUES SUBSTITUÉES PAR HÉTÉROARYLE À ACTIVITÉ HERBICIDE OU DÉRIVÉS DE CELLES-CI申请人:SYNGENTA LTD公开号:WO2011012862A1公开(公告)日:2011-02-03The invention relates to a compound of formula (I), which is suitable for use as a herbicide wherein G is hydrogen or an agriculturally acceptable metal, sulfonium, ammonium or latentiating group; Q is a unsubstituted or substituted C3-C8 saturated or mono-unsaturated heterocyclyl containing at least one heteroatom selected from O, N and S, or Q is heteroaryl or substituted heteroaryl; m is 1, 2 or 3; and Het is an optionally substituted monocyclic or bicyclic heteroaromatic ring; and wherein the compound is optionally an agronomically acceptable salt thereof.
-
TRIAZOLE ACC INHIBITORS AND USES THEREOF
表征谱图
-
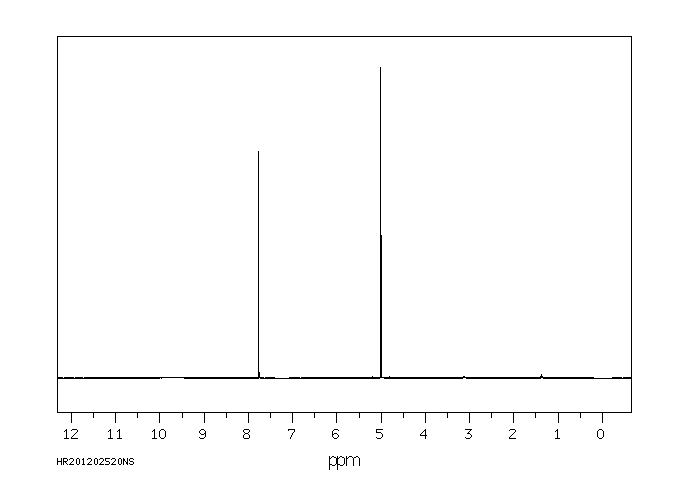
氢谱1HNMR
-
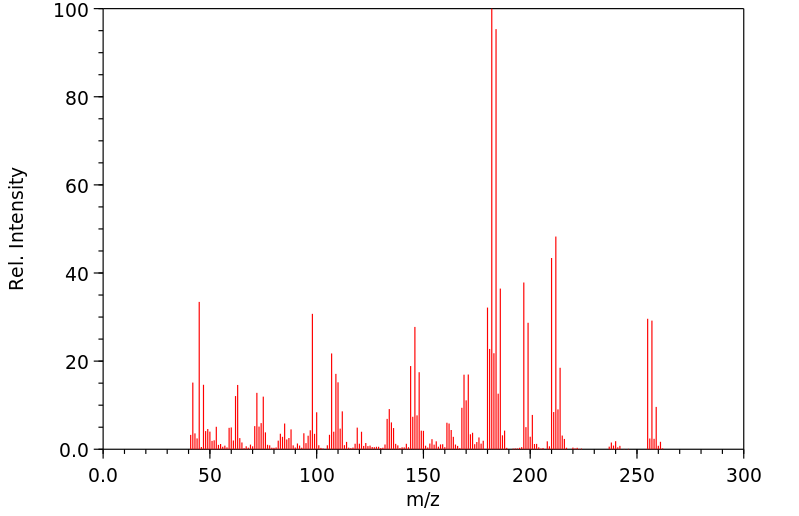
质谱MS
-
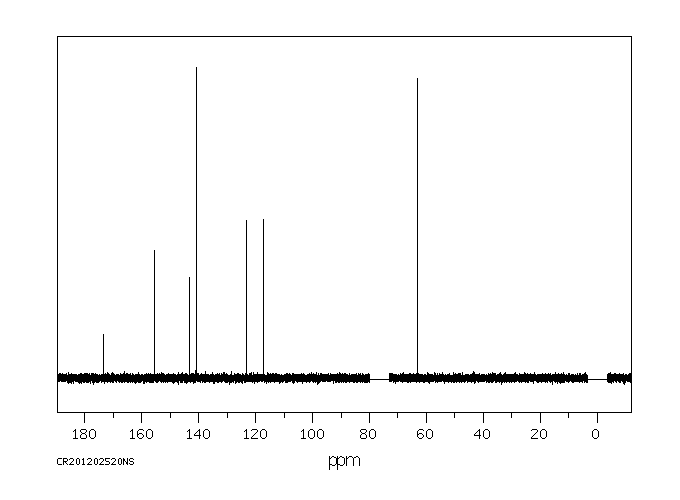
碳谱13CNMR
-
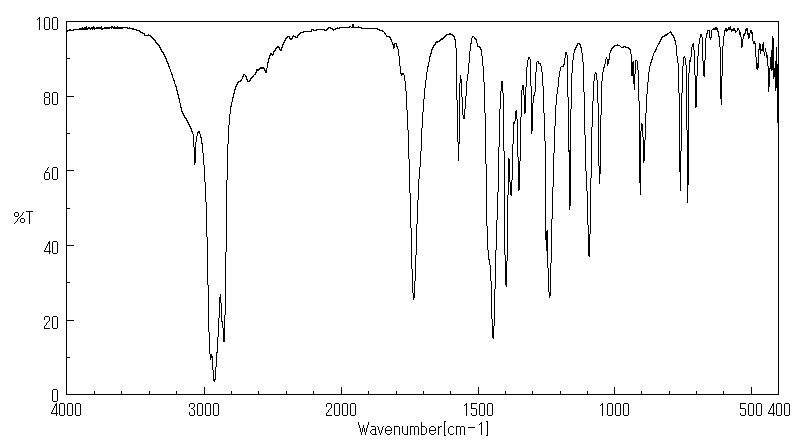
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息