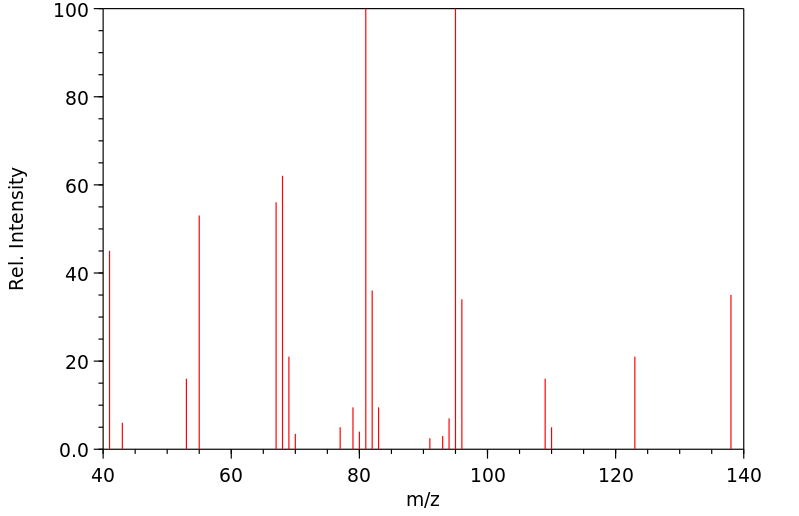
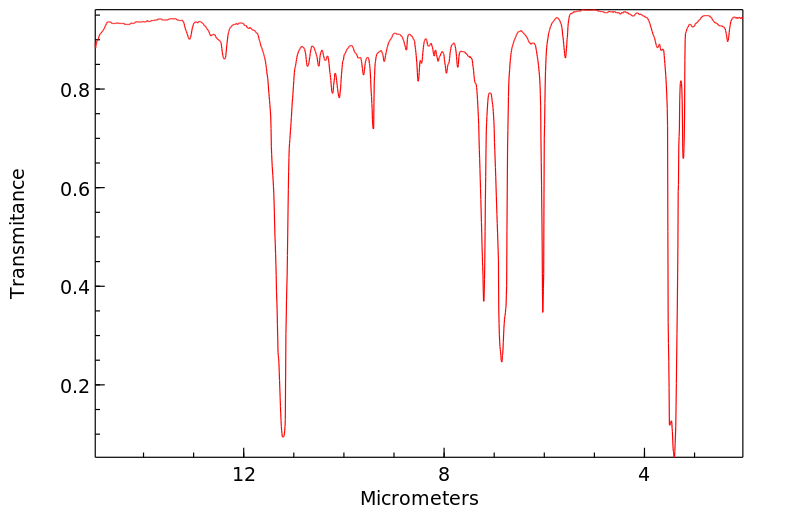
Cyclohexane, 1-methyl-4-(1-methylethenyl)-, cis- | 1879-07-8
中文名称
——
中文别名
——
英文名称
Cyclohexane, 1-methyl-4-(1-methylethenyl)-, cis-
英文别名
cis-p-8-menthene;cis-p-menth-8-ene;cis-8-p-menthene;cis-p-meth-8-ene;cis-p-menth-8-ene;cis-p-Menth-8-en;cis-1-methyl-4-(1-methylethenyl)-cyclohexane
CAS
1879-07-8
化学式
C10H18
mdl
——
分子量
138.253
InChiKey
WPMKLOWQWIDOJN-AOOOYVTPSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:174-175 °C
-
密度:0.8320 g/cm3
计算性质
-
辛醇/水分配系数(LogP):3.39
-
重原子数:10.0
-
可旋转键数:1.0
-
环数:1.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:0.0
-
氢给体数:0.0
-
氢受体数:0.0
反应信息
-
作为反应物:描述:Cyclohexane, 1-methyl-4-(1-methylethenyl)-, cis- 、 乙腈 在 硫酸 作用下, 反应 48.0h, 以10%的产率得到N-cis-p-menth-8-ylacetamide参考文献:名称:Kozlov, N. G.; Popova, L. A.; Novikova, M. G., Journal of Organic Chemistry USSR (English Translation), 1986, vol. 22, p. 477 - 480摘要:DOI:
-
作为产物:描述:alkaline earth salt of/the/ methylsulfuric acid 生成 Cyclohexane, 1-methyl-4-(1-methylethenyl)-, cis-参考文献:名称:The Isomerization of 8(9)-p-Menthene1,2摘要:DOI:10.1021/ja01163a050
文献信息
-
Photochemical transformations—II作者:K.M. Saplay、Ranjana Sahni、N.P. Damodaran、Sukh DevDOI:10.1016/0040-4020(80)85062-9日期:1980.1Experimental parameters governing π-electrons participation during photolysis of citronellyl iodide have been investigated. Photoproducts resulting from irradiation of 2,3-dihydro-6(Z)- and 2,3-dihydro-6(E)-farnesyl iodides have been characterised.
-
Thermal Isomerization of (+)-<i>cis</i>- and (−)-<i>trans</i>-Pinane Leading to (−)-β-Citronellene and (+)-Isocitronellene作者:Achim Stolle、Bernd Ondruschka、Werner Bonrath、Thomas Netscher、Matthias Findeisen、Markus M. HoffmannDOI:10.1002/chem.200800298日期:2008.7.28Catalyzed and uncatalyzed rearrangement reactions of terpenoids play a major role in laboratory and industrial-scale synthesis of fine chemicals. Herein, we present our results on the thermally induced isomerization of pinane (1). Investigation of the thermal behavior of (+)-cis- (1 a) and (-)-trans-pinane (1 b) in a flow-type reactor reveals significant differences in both reactivity and selectivity concerning
-
Subbarao, Kanury V.; Damodaran, N. P.; Dev, Sukh, Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 1987, vol. 26, # 1-12, p. 1008 - 1011作者:Subbarao, Kanury V.、Damodaran, N. P.、Dev, SukhDOI:——日期:——
-
Kalechits, G. V.; Rusak, M. F., Journal of general chemistry of the USSR, 1986, vol. 56, p. 1881 - 1884作者:Kalechits, G. V.、Rusak, M. F.DOI:——日期:——
-
Equilibrium transformations of p-menthenes作者:Z. A. Filippenko、G. N. Roganov、V. V. Bazyl'chikDOI:10.1007/bf00598205日期:1988.11
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸