(E)-3-(4-fluorophenyl)-1-(4-(methylthio)phenyl)prop-2-en-1-one | 126443-15-0
分子结构分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.7
-
重原子数:19
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.06
-
拓扑面积:42.4
-
氢给体数:0
-
氢受体数:3
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-甲硫基苯乙酮 4-(Methylthio)acetophenone 1778-09-2 C9H10OS 166.244
反应信息
-
作为反应物:描述:(E)-3-(4-fluorophenyl)-1-(4-(methylthio)phenyl)prop-2-en-1-one 在 dipotassium peroxodisulfate 、 氰化钠 、 ammonium acetate 、 溶剂黄146 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 生成 L 167307参考文献:名称:Potent, orally absorbed glucagon receptor antagonists摘要:The SAR of 2-pyridyl-3,5-diaryl pyrroles, ligands of the human glucagon receptor and inhibitors of p38 kinase, were investigated. This effort resulted in the identification of 2-(4-pyridyl)-5-(4-chlorophenyl)-3-(5-bromo-2-propyloxyphenyl)pyrrole 49 (L-168,049), a potent (Kb = 25 nM), selective antagonist of glucagon. (C) 1999 Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0960-894x(99)00081-5
-
作为产物:描述:茴香硫醚 在 sodium hydroxide 、 三氯氧磷 作用下, 以 甲醇 、 氯苯 为溶剂, 反应 2.0h, 生成 (E)-3-(4-fluorophenyl)-1-(4-(methylthio)phenyl)prop-2-en-1-one参考文献:名称:一些含 4'-甲硫基的芳基和芳基呋喃基吡唑啉的简便合成及其抗菌活性研究摘要:4-乙酰苯硫醚与不同的芳基和芳基呋喃醛在羟醛条件下缩合得到 α, β-不饱和酮(丙烯酮),它们与肼进行简单而干净的环化,以定量收率得到吡唑啉(4-6)。新合成化合物的结构已在光谱研究的基础上得到确认。测试了所有新合成的化合物的抗菌和抗真菌活性。DOI:10.1080/10426500500328939
文献信息
-
Trisulfur-Radical-Anion-Triggered C(sp<sup>2</sup>)–H Amination of Electron-Deficient Alkenes作者:Khang X. Nguyen、Phuc H. Pham、Thao T. Nguyen、Chou-Hsun Yang、Hoai T. B. Pham、Tung T. Nguyen、Haobin Wang、Nam T. S. PhanDOI:10.1021/acs.orglett.0c03846日期:2020.12.18A trisulfur-radical-anion (S3̇–)-triggered C(sp2)–H amination of α,β-unsaturated carbonyl derivatives with simple amines has been demonstrated. This protocol provides convenient access to a variety of synthetically valuable N-unprotected and secondary β-enaminones with absolute Z selectivity and tertiary β-enaminones with E selectivity. Mechanistic probe and electronic structure theory calculations
-
Replacement of Chalcone-Ethers with Chalcone-Thioethers as Potent and Highly Selective Monoamine Oxidase-B Inhibitors and Their Protein-Ligand Interactions作者:Bijo Mathew、Jong Min Oh、Ahmed Khames、Mohamed A. Abdelgawad、T. M. Rangarajan、Lekshmi R. Nath、Clement Agoni、Mahmoud E. S. Soliman、Githa Elizabeth Mathew、Hoon KimDOI:10.3390/ph14111148日期:——
To develop new potent and highly selective MAO-B inhibitors from chalcone-thioethers, eleven chalcones-thioethers were synthesized and their monoamine oxidase (MAO) inhibition, kinetics, reversibility, and cytotoxicity of lead compounds were analyzed. Molecular dynamics were carried out to investigate the interactions. Compound TM8 showed potent inhibitory activity against MAO-B, with an IC50 value of 0.010 µM, followed by TM1, TM2, TM7, and TM10 (IC50 = 0.017, 0.021, 0.023, and 0.026 µM, respectively). Interestingly, TM8 had an extremely high selectivity index (SI; 4860) for MAO-B. Reversibility and kinetic experiments showed that TM8 and TM1 were reversible and competitive inhibitors of MAO-B with Ki values of 0.0031 ± 0.0013 and 0.011± 0.001 µM, respectively. Both TM1 and TM8 were non-toxic to Vero cells with IC50 values of 241.8 and 116.3 µg/mL (i.e., 947.7 and 402.4 µM), respectively, and at these IC50 values, both significantly reduced reactive oxygen species (ROS) levels. TM1 and TM8 showed high blood-brain barrier permeabilities in the parallel artificial membrane permeability assay. Molecular dynamics studies were conducted to investigate interactions between TM1 and TM8 and the active site of MAO-B. Conclusively, TM8 and TM1 are potent and highly selective MAO-B inhibitors with little toxicity and good ROS scavenging abilities and it is suggested that both are attractive prospective candidates for the treatment of neurological disorders.
为了从查尔酮硫醚中开发新的强效、高选择性 MAO-B 抑制剂,我们合成了 11 种查尔酮硫醚,并分析了它们对单胺氧化酶(MAO)的抑制作用、动力学、可逆性以及先导化合物的细胞毒性。此外,还进行了分子动力学研究以探究其相互作用。化合物 TM8 对 MAO-B 具有强效抑制活性,IC50 值为 0.010 µM,其次是 TM1、TM2、TM7 和 TM10(IC50 分别为 0.017、0.021、0.023 和 0.026 µM)。有趣的是,TM8 对 MAO-B 具有极高的选择性指数(SI;4860)。可逆性和动力学实验表明,TM8 和 TM1 是 MAO-B 的可逆竞争性抑制剂,Ki 值分别为 0.0031 ± 0.0013 和 0.011± 0.001 µM。TM1 和 TM8 对 Vero 细胞均无毒性,IC50 值分别为 241.8 和 116.3 µg/mL(即 947.7 和 402.4 µM)。在平行人工膜渗透性试验中,TM1 和 TM8 显示出较高的血脑屏障渗透性。分子动力学研究调查了 TM1 和 TM8 与 MAO-B 活性位点之间的相互作用。结果表明,TM8 和 TM1 是强效、高选择性的 MAO-B 抑制剂,毒性小,具有良好的清除 ROS 能力。 -
CHALCONE DERIVATIVE COMPOUNDS申请人:NIPPON OIL AND FATS COMPANY, LIMITED公开号:EP0328669A1公开(公告)日:1989-08-23Chalcone derivative compounds represented by general formula (I) or (II), wherein R₁ and R₂ each represents a halogen atom, a hydroxy group, an amino group, a dimethylamino group, a nitro group, a cyano group, a phenyl group, an acetyl group, an alkyl group containing 1 to 18 carbon atoms or an alkyloxy group containing 1 to 22 carbon atoms, n₁ and n₂ each represents an integer of 0 to 21, and m₁ and m₂ each represents an integer of 0 to 5.
-
Holla, B. Shivarama; Mahalinga; Poojary, Boja, Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 2006, vol. 45, # 2, p. 568 - 571作者:Holla, B. Shivarama、Mahalinga、Poojary, Boja、Ashok, Mithun、AkberaliDOI:——日期:——
-
‘On water’ synthesis of 2,4-diaryl-2,3-dihydro-1,5-benzothiazepines catalysed by sodium dodecyl sulfate (SDS)作者:Gaurav Sharma、Raj Kumar、Asit K. ChakrabortiDOI:10.1016/j.tetlet.2008.04.146日期:2008.6An efficient synthesis of 1,3-diaryl-2,3-dihydro-1,5-benzothiazepines has been developed by the reaction of various 1,3-diaryl-2-propenones with 2-aminothiophenol in water under neutral conditions catalysed by SDS. Excellent chemoselectivity was observed for substrates possessing halogen atoms or nitro/alkoxy/thioalkyl groups which did not undergo competitive aromatic nucleophilic substitution of the halogen atoms or the nitro group, reduction of the nitro or the a,p-unsaturated carbonyl group, or dealkylation of the alkoxy/thioalkoxy groups. (C) 2008 Elsevier Ltd. All rights reserved.
表征谱图
-
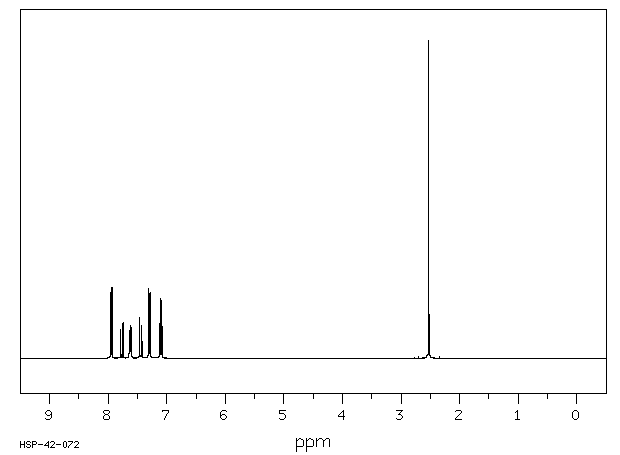
氢谱1HNMR
-
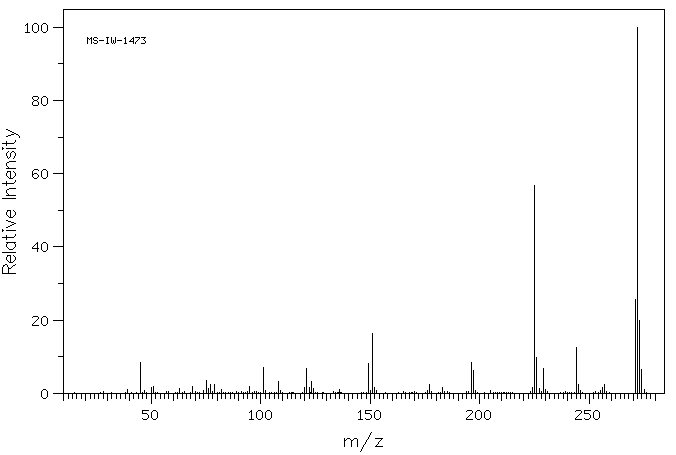
质谱MS
-
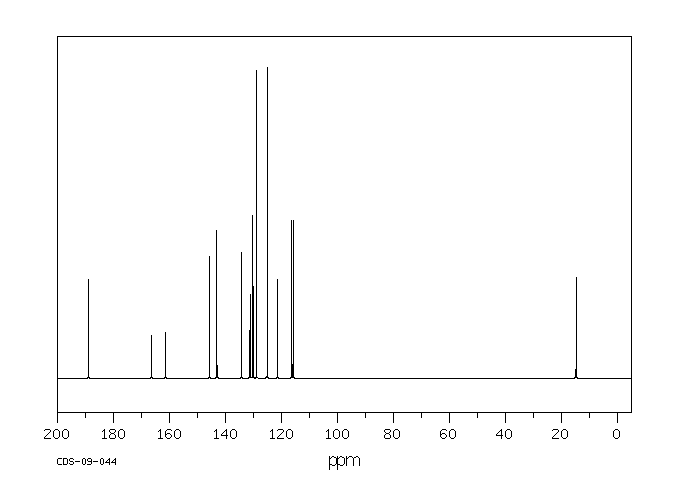
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息