(E)-3-[4-(2-methoxycarbonylvinyl)phenyl]acrylic acid methyl ester | 23746-55-6
中文名称
——
中文别名
——
英文名称
(E)-3-[4-(2-methoxycarbonylvinyl)phenyl]acrylic acid methyl ester
英文别名
(2E,2E’)-dimethyl 3,3’-(1,4-phenylene)diacrylate;dimethyl 3,3′-(1,4-phenylene)(2E,2′E)-diacrylate;(2E,2'E)-dimethyl 3,3'-(1,4-phenylene)diprop-2-enoate;dimethyl (2E,2'E)-3,3'-(1,4-phenylene)diprop-2-enoate;(2E,2E')-dimethyl 3,3'-(1,4-phenylene)diacrylate;3,3'-(1,4-phenylene)(2E,2'E)diacrylate;Dimethyl 1,4-Phenylenediacrylate;methyl (E)-3-[4-[(E)-3-methoxy-3-oxoprop-1-enyl]phenyl]prop-2-enoate
CAS
23746-55-6
化学式
C14H14O4
mdl
——
分子量
246.263
InChiKey
IFNSXAJHSAPYLB-FIFLTTCUSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:18
-
可旋转键数:6
-
环数:1.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:52.6
-
氢给体数:0
-
氢受体数:4
上下游信息
反应信息
-
作为反应物:描述:(E)-3-[4-(2-methoxycarbonylvinyl)phenyl]acrylic acid methyl ester 在 palladium on activated charcoal 氢气 作用下, 以 甲醇 为溶剂, 反应 6.0h, 以100%的产率得到dimethyl 3,3'-(1,4-phenylene)dipropanoate参考文献:名称:过渡金属催化的二羰基底物的化学选择性甲基化。摘要:与三甲基甲硅烷基重氮甲烷,三苯基膦和2-丙醇的铑和铜催化的亚甲基化反应用于在也含有较低反应性羰基的底物中与醛,烷氧基甲基酮和三氟甲基酮发生化学选择性反应。以高收率获得末端烯烃,并且在亚甲基化过程中不需要保护基。DOI:10.1021/jo800777w
-
作为产物:参考文献:名称:化学计量比的吡啶N-氧化物和NaBH 4促进的Fischer烷氧基卡宾配合物的氧化脱金属-硫和硒促进了Fischer亚氨基卡宾配合物的脱金属摘要:在温和的条件下,用化学计量的吡啶N-氧化物(PNO)系统研究了Fischer烷氧基卡宾卡宾配合物的氧化,从相应的单卡宾配合物形成酯产物,收率良好。Fischer烷氧基双卡宾络合物在受控条件下有效地进行了逐步氧化脱金属,分别生成了酯-单卡宾和二酯产品。该氧化方案证明了在温和条件下氧化费歇尔烷氧基卡宾卡宾配合物的一般有效方法,为从费希尔双卡宾配合物制得新型单卡宾配合物提供了一条新途径。在NaBH 4存在下通过在Fischer卡宾络合物的MC键中插入硫或硒原子,费托亚氨基卡宾络合物与乙醇中的元素硫或硒在环境温度下反应选择性地生成硫酮或Selone络合物,还获得了无金属的Selone。亚氨基卡宾配合物和selone衍生物的分子结构通过X射线晶体学研究证实。NaBH 4促进的脱金属方案表明了将Fischer氨基卡宾配合物脱金属的潜在新途径。DOI:10.1016/j.jorganchem.2006.05.021
文献信息
-
Palladium-Catalyzed Alkoxycarbonylation of Terminal Alkenes To Produce α,β-Unsaturated Esters: The Key Role of Acetonitrile as a Ligand作者:Andrei V. Malkov、Nolwenn Derrien、Maciej Barłóg、Pavel KočovskýDOI:10.1002/chem.201304798日期:2014.4.14A mild protocol has been developed for the PdII‐catalyzed alkoxycarbonylation of terminal olefins to produce α,β‐unsaturated esters with a wide range of substrates. Key features are the use of MeCN as solvent (and/or ligand) to control the reactivity of the intermediate Pd complexes and the combination of CO with O2, which facilitates the CuII‐mediated reoxidation of the Pd0 complex to PdII and prevents已开发出一种温和的方案,用于末端烯烃的Pd II催化的烷氧羰基化反应,以生产具有多种底物的α,β-不饱和酯。关键特征是使用MeCN作为溶剂(和/或配体)来控制中间Pd配合物的反应性,以及CO与O 2的组合,这有助于Cu II介导的Pd 0配合物向Pd II和Pd II的再氧化。防止双羰基化。
-
Synthesis of a Novel <i>C</i><sub>2</sub>-Symmetric Thiourea and Its Application in the Pd-Catalyzed Cross-Coupling Reactions with Arenediazonium Salts under Aerobic Conditions作者:Mingji Dai、Bo Liang、Cuihua Wang、Jiahua Chen、Zhen YangDOI:10.1021/ol036182u日期:2004.1.1[reaction: see text] A novel thiourea-based C2-symmetric ligand was synthesized, and its application in the palladium-catalyzed Heck and Suzuki coupling reactions of arenediazonium salts was evaluated. The reactions, which were performed at room temperature, without added base, and under aerobic conditions, produced product in 4 h with good yield. The corresponding arenediazonium salts were easily
-
High Loading of Pd Nanoparticles by Interior Functionalization of MOFs for Heterogeneous Catalysis作者:Bappaditya Gole、Udishnu Sanyal、Rahul Banerjee、Partha Sarathi MukherjeeDOI:10.1021/acs.inorgchem.5b02739日期:2016.3.7agglomeration upon increasing their loading amount into metal–organic frameworks (MOFs) has been addressed by functionalization of MOFs with alkyne groups. The alkynophilicity of the Pd2+ (or other noble metals) ions has been utilized successfully for significant loading of Pd NPs into alkyne functionalized MOFs. It has been shown here that the size and loading amount of Pd NPs are highly dependent在本报告中,已通过将炔烃基团官能化,解决了纳米颗粒(NP)在金属有机骨架(MOF)中的负载量增加时的团聚问题。Pd 2+(或其他贵金属)离子的亲核性已成功用于Pd NP大量装载到炔烃官能化的MOF中。此处已表明,Pd NP的大小和负载量高度依赖于MOF的表面积和孔宽度。Pd NP的负载量单调增加,而不会改变其在特定MOF上的大小分布。重要的是,炔烃基对于Pd 2+的独特作用通过进行考虑到没有炔烃部分的MOF的对照实验也证明了稳定性。NP的制备涉及两个不同的步骤,即。MOF内部金属离子的吸附和金属离子的还原。这两个步骤均通过显微镜技术进行监控。该报告还证明了[电子邮件保护的] NPs是分别用于芳基溴化物或碘化物和烯烃的Heck偶联和加氢反应的极其有效的非均相催化剂的适用性。
-
Preparation, Properties, and Structures of Pentanuclear [{Ni<sub>2</sub>L}<sub>2</sub>(μ-csalen)<i>M</i>]<sup>2+</sup>Complexes (L = Macrocyclic N<sub>6</sub>S<sub>2</sub>Donor Ligand)作者:Matthias Golecki、Berthold KerstingDOI:10.1002/zaac.201400427日期:2015.2The dinuclear nickel complex [Ni2L(μ-Cl)]+ (1), where L2– is a 24-membered macrocyclic N6S2 ligand, reacts readily with 3-formyl-4-hydroxy-benzoic acid (Hfhba) to form the carboxylato-bridged complex [Ni2L(μ-fhba)]+ (2). Complex 2 undergoes a condensation reaction with ethylene diamine to produce a tetranuclear complex [Ni2L}2(μ-H2csalen)]2+ (3), in which two dinuclear Ni2L} units are bridged via双核镍络合物 [Ni2L(μ-Cl)]+ (1),其中 L2– 是 24 元大环 N6S2 配体,很容易与 3-甲酰基-4-羟基-苯甲酸 (Hfhba) 反应形成羧基-桥接复合物 [Ni2L(μ-fhba)]+ (2)。配合物 2 与乙二胺发生缩合反应,生成四核配合物 [Ni2L}2(μ-H2csalen)]2+ (3),其中两个双核 Ni2L} 单元通过 csalen 配体的去质子化羧酸官能团桥接N,N'-双(4-羧基水杨基)-1, 2-二氨基乙烷。同样的化合物也可以直接由 1 和 H2csalen 制备。3 与 NiCL2·6 、Cu(OAc)2·H2O 或 Pd(OAc)2 的络合提供 [Ni2L}2(μ-csalen)M]2+ [M = Ni (4a) 类型的五核配合物,铜(4b),钯(4c)]。所有复合物均以高氯酸盐的形式分离,并通过 ESI-MS、红外和紫外/可见光谱进行研究。4c
-
An <i>in Situ</i> Generated Palladium on Aluminum Oxide: Applications in Gram-Scale Matsuda–Heck Reactions作者:Simon Pape、Lauryna Daukšaitė、Sandra Lucks、Xiaoting Gu、Heiko BrunnerDOI:10.1021/acs.orglett.6b03268日期:2016.12.16In situ generated palladium on aluminum oxide provides an active catalytic system for Matsuda-Heck reactions in gram-scale. The novel catalyst proceeded through a significantly higher catalytic activity compared to the classical Pd/C system. Based on the high catalytic activity the first α,β,β-triarylation of methyl acrylate in good yields could be provided in one-step.
表征谱图
-
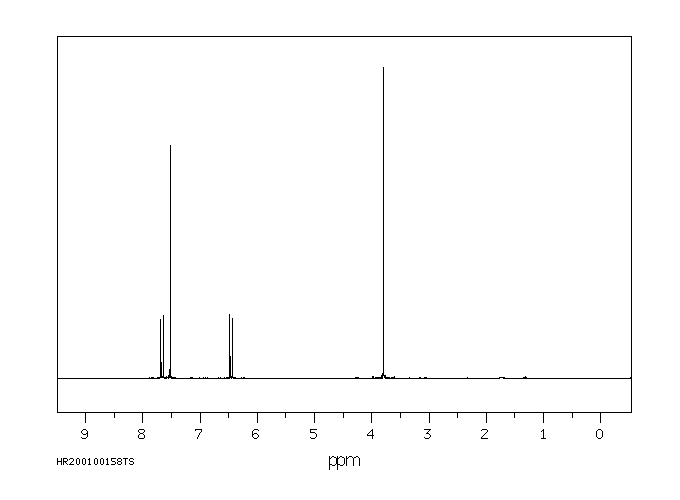
氢谱1HNMR
-
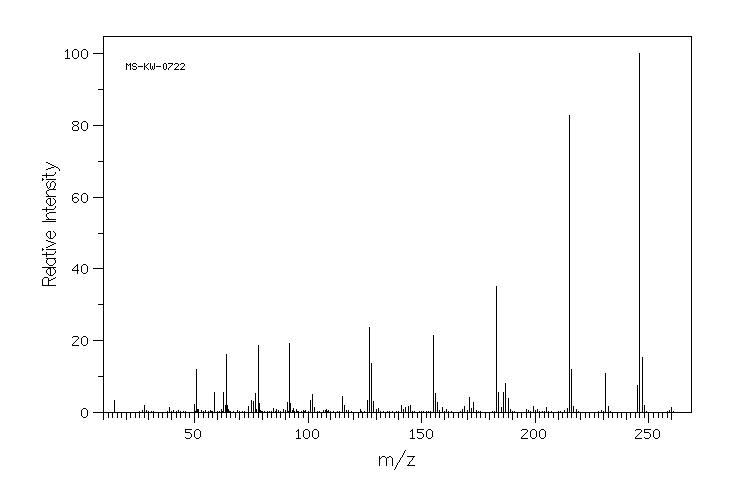
质谱MS
-
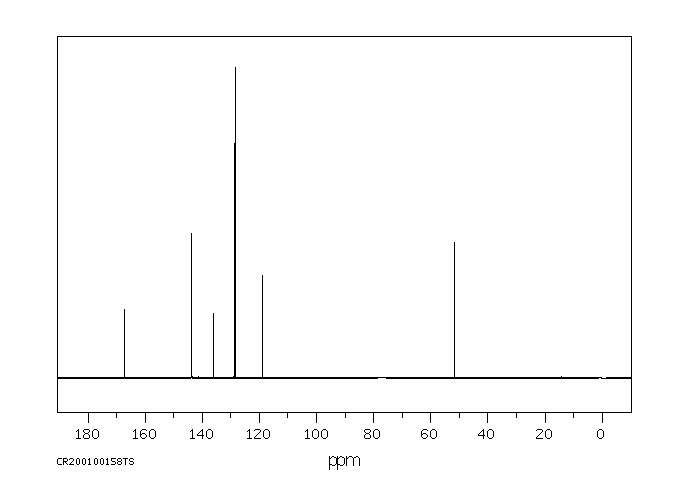
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(E)-3-(4-(叔丁基)苯基)丙烯酸乙酯
(E)-3-(2-(三氟甲基)苯基)丙烯酸乙酯
(E)-3-(2,4-二甲氧基苯基)丙烯酸乙酯
(2E)-N-[2-(3-羟基-2-氧代-2,3-二氢-1H-吲哚-3-基)乙基]-3-苯基丙-2-烯酰胺
黄金树苷
鲁索曲波帕
香豆酸肉桂酯
香豆酰多巴胺
香草醛缩丙酮
顺式邻羟基肉桂酸
顺式芥子酸
顺式-曲尼司特
顺式-乙基肉桂酸酯
顺式-N-阿魏酰酪胺
顺式-3,4-二甲氧基苯丙烯酸
顺式-2-((叔丁氧羰基)氨基)-3-(4-氨甲酰基-2,6-二甲苯基)丙烯酸甲酯
顺-o-羧基肉桂酸
顺-2-甲氧基肉桂酸
阿魏酸钠
阿魏酸酰胺
阿魏酸甲酯
阿魏酸甲酯
阿魏酸甲酯
阿魏酸松柏酯
阿魏酸杂质1
阿魏酸异辛酯
阿魏酸哌嗪
阿魏酸二十烷基酯
阿魏酸乙酯
阿魏酸4-O-硫酸二钠盐
阿魏酸-D3
阿魏酸
阿魏酸
阿魏酰酪胺
间羟基肉桂酸
间羟基肉桂酸
间硝基肉桂酸
间甲基肉桂酸
间甲基反式肉桂酸甲酯
间氯肉桂酸
间三氟甲氧基肉桂酸甲酯
间-香豆酸
间-(三氟甲基)-肉桂酸
锂(E)-2-溴-3-苯基丙烯酸酯
钠二乙基2-[(氧代氨基)-苯基亚甲基]丙二酸酯盐
酪氨酸磷酸化抑制剂AG 556
酪氨酸磷酸化抑制剂AG 527
酪氨酸磷酸化抑制剂AG 490
酪氨酸磷酸化抑制剂A46
酪氨酸磷酸化抑制剂 AG 30