cis-cyclopentane-1,3-diol | 16326-97-9
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:31°C
-
沸点:151.45°C (rough estimate)
-
密度:1.0990
计算性质
-
辛醇/水分配系数(LogP):-0.2
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:40.5
-
氢给体数:2
-
氢受体数:2
安全信息
-
海关编码:2906199090
-
储存条件:存储条件:2-8°C,密封保存。
SDS
上下游信息
反应信息
-
作为反应物:描述:cis-cyclopentane-1,3-diol 在 氧气 、 potassium carbonate 作用下, 以 水 为溶剂, 120.0 ℃ 、206.85 kPa 条件下, 反应 72.0h, 以97%的产率得到(S)-3-hydroxycyclopentanone参考文献:名称:催化不对称氧化反应中的手性取代聚-N-乙烯基吡咯烷酮和双金属纳米团簇摘要:由L-氨基酸合成了在吡咯烷酮环的C5处含有不对称中心的一类新的聚-N-乙烯基吡咯烷酮。这些聚合物,特别是17,用于稳定纳米团簇,例如Pd/Au,用于1,3-和1,2-环烷二醇和烯烃的催化不对称氧化,而Cu/Au用于环烷烃的CH氧化。研究发现,吡咯烷酮环中的C5取代基体积越大,产生的光学产率越大。 (±)-1,3- 和 1,2-反式环烷二醇的氧化动力学拆分以及内消旋顺式二醇的去对称化均在水中的氧气气氛下用 0.15 mol% Pd/Au (3:1)-17 进行,提供优异的 (S)-羟基酮的化学和光学收率。在 30 psi 氧气水中,用 0.5 mol% Pd/Au (3:1)-17 氧化各种烯烃,得到 >93% ee 的二羟基化产物。 (R)-柠檬烯在 25 °C 下在 C-1,2-环烯官能团处发生氧化,产生 (1S,2R,4R)-二羟基柠檬烯 49,产率 92%。重要的是,环烷烃用乙腈中的DOI:10.1021/jacs.6b12113
-
作为产物:描述:rac-3-羟基环戊酮 在 1-hydroxy-6,8-diphenyl-1,2,3,4-tetrahydro-2-oxa-1-boranaphthalene 、 三乙基硼氢化锂 作用下, 生成 cis-cyclopentane-1,3-diol参考文献:名称:在三联苯硼酸的帮助下将β-羟基酮立体选择性还原为1,3-二醇摘要:1-Hydroxy-6,8-diphenyl-1,2,3,4-tetrahydro-2-oxa-1-boranaphthalene(三联苯硼酸 1)用于立体选择性还原无环和环状 β-羟基酮。三联苯硼酸 1 和无环 β-羟基酮 2 通过共沸去除水转化为相应的硼酸酯。所得硼酸酯用还原剂原位处理以几乎完全得到syn 1,3-二醇3。抗 α-取代的 β-羟基酮 8 也被还原以立体选择性地产生抗、抗 1,3-二醇 9。此外,3-羟基-1-环戊酮的还原以高选择性得到顺式二醇11。DOI:10.1246/cl.1996.539
文献信息
-
SYNTHESIS AND APPLICATION OF CHIRAL SUBSTITUTED POLYVINYLPYRROLIDINONES申请人:Kansas State University Research Foundation公开号:US20200306737A1公开(公告)日:2020-10-01Chiral polyvinylpyrrolidinone (CSPVP), complexes of CSPVP with a core species, such as a metallic nanocluster catalyst, and enantioselective oxidation reactions utilizing such complexes are disclosed. The CSPVP complexes can be used in asymmetric oxidation of diols, enantioselective oxidation of alkenes, and carbon-carbon bond forming reactions, for example. The CSPVP can also be complexed with biomolecules such as proteins, DNA, and RNA, and used as nanocarriers for siRNA or dsRNA delivery.
-
The utility of the dehalogenation–deetherification sequence for the proof of structure of methoxyhalocyclohexanols and methoxyhalocyclopentanols. Synthesis of the <i>cis-</i> and <i>trans-</i>2- and -3-methoxy-cyclohexanols and -cyclopentanols作者:R. A. B. Bannard、A. A. Casselman、E. J. Langstaff、R. Y. MoirDOI:10.1139/v67-423日期:1967.11.1
An unequivocal proof of structure for the methoxychlorocyclopentanols (I′c–IV′c) was obtained by deetherification with 68% hydrobromic acid at 65–70°, followed by hydrogenolysis with Raney nickel and hydrogen, to the 1,2- and 1,3-cyclopentanediols, in the same manner as the methoxybromocyclohexanols (I–IV) were converted into the 1,2- and 1,3-cyclohexanediols. Hydrogenolysis of the methoxybromocyclohexanols and the methoxychlorocyclopentanols provided stereospeciflc syntheses for the cis- and trans-2- and -3-methoxycy-clohexanols and -cyclopentanols in 80–97% yields. Deetherification of the latter compounds with 68% hydrobromic acid gave the corresponding 1,2- and 1,3-cyclohexanediols and 1,2-cyclopentanediols in 70–90% yields, but only 5–7% yields of the 1,3-cyclopentanediols. For the proof of structure of methoxyhalocyclanols, deetherification should therefore precede, rather than follow, dehalogenation.
-
CoTPP-catalyzed reaction of saturated bicyclic endoperoxides作者:Metin Balci、Nihat AkbulutDOI:10.1016/s0040-4020(01)96533-0日期:1985.1Saturated bicyclic endoperoxides were exposed to CoTPP (Cobalt-meso-tetraphenylporphyrine) in chloroform, the peroxide bond was cleaved and a mixture of products arising from fragmentation and reduction obtained.
-
Chemoselective formation of cyclo-aliphatic and cyclo-olefinic 1,3-diols <i>via</i> pressure hydrogenation of potentially biobased platform molecules using Knölker-type catalysts作者:Christian A. M. R. van Slagmaat、Teresa Faber、Khi Chhay Chou、Alfonso J. Schwalb Freire、Darya Hadavi、Peiliang Han、Peter J. L. M. Quaedflieg、Gerard K. M. Verzijl、Paul L. Alsters、Stefaan M. A. De WildemanDOI:10.1039/d1dt01252e日期:——
Hemicellulose-derived five-membered cyclic ketones are strategic precursors for sustainable 1,3-diol building blocks, and bifunctional iron catalysts provide unprecedented chemoselectivity in hydrogenation toward aliphatic and olefinic structures.
-
Chemical-Vapor-Deposited Materials for High Thermal Conductivity Applications作者:Jitendra S. Goela、Nathaniel E. Brese、Michael A. Pickering、John E. GraebnerDOI:10.1557/mrs2001.116日期:2001.6
Chemical vapor deposition (CVD) is an attractive method for producing bulk and thin-film materials for a variety of applications. In this method, gaseous reagents condense onto a substrate and then react to produce solid materials. The materials produced by CVD are theoretically dense, highly pure, and have other superior properties.
化学气相沉积(CVD)是一种用于生产各种应用的块状和薄膜材料的吸引人的方法。在这种方法中,气态试剂凝结在基底上,然后发生反应产生固体材料。CVD生产的材料在理论上是致密的、高纯度的,并具有其他优越的性能。
表征谱图
-
氢谱1HNMR
-
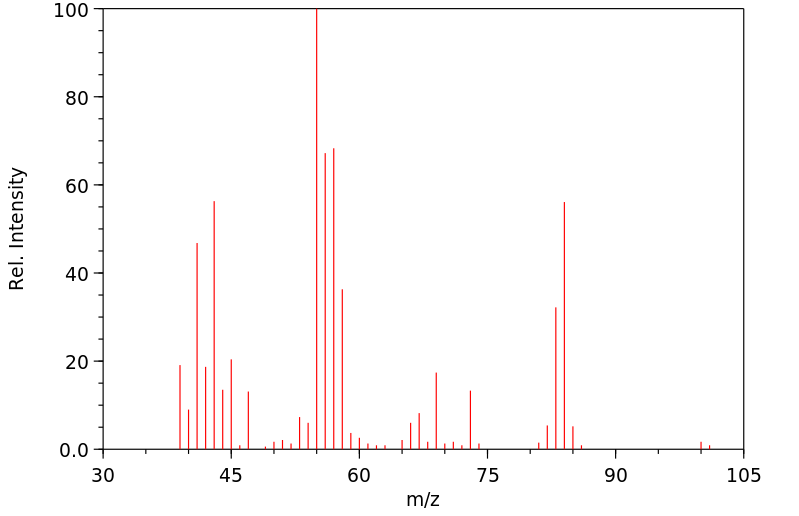
质谱MS
-
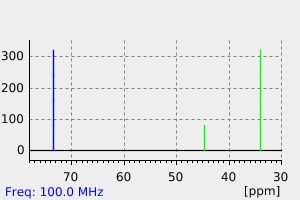
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息