(E)-5-methyl-2-hexene | 7385-82-2
中文名称
——
中文别名
——
英文名称
(E)-5-methyl-2-hexene
英文别名
trans-5-Methyl-hexen-(2);5-Methyl-trans-hexen-(2);5-Methyl-2-hexene;(E)-5-methylhex-2-ene
CAS
7385-82-2
化学式
C7H14
mdl
——
分子量
98.1882
InChiKey
GHBKCPRDHLITSE-SNAWJCMRSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-124.34°C
-
沸点:88.11°C
-
密度:0.6883
-
保留指数:679;661
计算性质
-
辛醇/水分配系数(LogP):2.9
-
重原子数:7
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.71
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2901299090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 5-甲基-1-己烯 5-methyl-1-hexene 3524-73-0 C7H14 98.1882
反应信息
-
作为反应物:参考文献:名称:1-亚甲基-2-乙烯基环丙烷及其衍生物的光谱表征摘要:描述和讨论了1-亚甲基-2-乙烯基环丙烷1及其一些衍生物的特征NMR和振动光谱性质。持续地在1745cm -1处发现了外循环CC拉伸振动。的1 1 H NMR谱表明,基本上独立于所述取代基的亚甲基和环质子间偶合图案。H-2和取代基的α-质子之间的偶合常数取决于构象。使用常见的取代基效应规则可轻松解释13 C化学位移和偶联常数。MNDO计算提供了质子-质子距离和键序,用于解释质子弛豫率和质子-质子耦合常数。DOI:10.1002/recl.19871060203
-
作为产物:描述:2-iodo-5-methyl-hexane 在 氢氧化钾 作用下, 生成 (E)-5-methyl-2-hexene参考文献:名称:Rohn, Justus Liebigs Annalen der Chemie, 1878, vol. 190, p. 312摘要:DOI:
文献信息
-
Dynamic π-Bonding of Imidazolyl Substituent in a Formally 16-Electron Cp*Ru(κ<sup>2</sup>-<i>P</i>,<i>N</i>)<sup>+</sup> Catalyst Allows Dramatic Rate Increases in (<i>E</i>)-Selective Monoisomerization of Alkenes作者:Erik R. Paulson、Curtis E. Moore、Arnold L. Rheingold、David P. Pullman、Ryan W. Sindewald、Andrew L. Cooksy、Douglas B. GrotjahnDOI:10.1021/acscatal.8b04345日期:2019.8.2in κ2-P,N coordination. For the first time, we show direct experimental evidence that the PN ligand has accepted a proton from the substrate by characterizing the intermediate Cp*Ru[η3-allyl][κ1-P)P–N+H], which highlights the essential role of the bifunctional ligand in promoting rapid and selective alkene isomerizations. Moreover, kinetic studies and computations reveal the role of alkene binding in烯烃异构化可以是一种原子经济的方法,可用于生成范围广泛的用于合成的烯烃中间体,但是完全平衡的二取代内部烯烃的混合物通常包含大量的位置异构体和几何异构体(E和Z)。用于烯烃异构化的大多数经典催化剂体系都难以动力学控制位置异构或E / Z异构。我们报告了配位不饱和,形式为16电子的Cp * Ru催化剂5,它可促进线性1-烯烃同时向其内部类似物进行区域和立体选择性异构化,从而提供(E)-2-烯烃大于95%。由于无腈催化剂5比以前发表的含腈类似物2 + 2a快400倍以上,因此在15分钟至4小时内完成5个完全环境温度反应的0.1-0.5摩尔%非常合理的负载量。的UV-vis,NMR,和计算研究描绘κ上膦作为hemilabile,四-电子给体的咪唑基片段2 - P,Ñ协调。对于第一次,我们显示直接的实验证据表明,PN配体已经通过表征中间的Cp *茹[η接受从基板的质子3 -烯丙基] [κ 1 - P)PN+
-
Catalyst versus Substrate Control of Forming (<i>E</i>)-2-Alkenes from 1-Alkenes Using Bifunctional Ruthenium Catalysts作者:Erik R. Paulson、Esteban Delgado、Andrew L. Cooksy、Douglas B. GrotjahnDOI:10.1021/acs.oprd.8b00315日期:2018.12.21Here we examine in detail two catalysts for their ability to selectively convert 1-alkenes to (E)-2-alkenes while limiting overisomerization to 3- or 4-alkenes. Catalysts 1 and 3 are composed of the cations CpRu(κ2-PN)(CH3CN)+ and Cp*Ru(κ2-PN)+, respectively (where PN is a bifunctional phosphine ligand), and the anion PF6–. Kinetic modeling of the reactions of six substrates with 1 and 3 generated在这里,我们详细研究了两种催化剂,它们能够选择性地将1-烯烃转化为(E)-2-烯烃,同时将过分异构化限制为3-或4-烯烃。催化剂1和3是由阳离子CPRU(κ的2 -PN)(CH 3 CN)+ 1和CP *茹(κ 2 -PN)+,分别为(其中,PN是双功能膦配体),和阴离子PF 6 –。六种底物与1和3的反应动力学模型生成第一和第二阶速率常数k 1和k 2(以及k3表示用于1-烯烃的转化反应的速率(适用时)ë)-2-烯(ķ 1),(ê)-2-烯烃至(Ë)-3-烯(ķ 2),和很快。所述ķ 1:ķ 2个比率计算以产生用于朝向与每个基底monoisomerization各催化剂选择性的量度。具有六个基板的1的k 1:k 2值在32到132范围内。3的k 1:k 2值在3个范围内它们对底物的依赖性更大,除5-hexen-2-one(k 1:k 2值仅为4.7)外,所有底物的依赖性都在192到62
-
Thermal reaction of azoisopropane in the presence of (E)-CH<sub>3</sub>CH CHCH<sub>3</sub>: reactions of the radical 2-Ċ<sub>3</sub>H<sub>7</sub>作者:László Seres、Ronald Fischer、Klaus Scherzer、Miklós GörgényiDOI:10.1039/ft9959101303日期:——The azoisopropane-initiated thermal reaction of (E)-CH3CHCHCH3 has been studied in the temperature range 489.5â542.0 K.For the reactions (CH3)2CHNNCH(CH3)2â 2 2-Ä3H7+ N2(1), 2-Ä3H7+(E)-CH3CHCHCH3â C3H8+(E)-Ä4H7(4), â(CH3)2CHCH(CH3)ÄH(CH3)(5), 2 2-Ä3H7â(CH3)2 CHCH(CH3)2(2) the following Arrhenius parameters were determined: log(k1/sâ 1)=(16.42 ± 0.30)â(201.9 ± 3.0) kJ molâ 1/θ, log[(k4/k1/22)/dm3/2 molâ 1/2 sâ 1/2]=(3.64 ± 0.40)â(46.9 ± 2.1) kJ molâ 1/θ, log[(k5/k1/22)/dm3/2 molâ 1/2 sâ 1/2](2.53 ± 0.60)â(39.5 ± 2.4) kJ molâ1/θ where θ=RT In 10.For the cross-combination ratios of the radicals 2-Ä3H7 and (Z)-Ä4H7, Ï[2-Ä3H7, (Z)-Ä4H7]t= 2.12 ± 0.10 was obtained, where the subscript t refers to the terminal combination.Formation of certain characteristic products was observed in various addition/isomerization/dissociation processes. 2-Ä3H7 addition to (E)-CH3CHCHCH3 is suggested as the rate-determining step, followed by 1, 4- and 1,5-H-atom shifts.在 489.5–542.0 K 温度范围内研究了 (E)- CHCH 偶氮异丙烷引发的热反应。对于反应 (CH3)2CHNNCH( )2−2 2-ä3H7+ N2(1), 2-3H7+(E)- CHCH —C3H8+(E)-4H7(4), —( )2CHCH( )—H( )(5), 2 2-3H7â ( )2 CHCH( )2(2) 确定下列阿累尼乌斯参数: log(k1/s−1)=(16.42 ± 0.30)−(201.9 ± 3.0) kJ mol− 1/θ, log[(k4/k1/22)/dm3/2 mol — 1/2 s — 1/2]=(3.64 ± 0.40) — (46.9 ± 2.1) kJ mol — 1/θ, log[(k5/k1/22)/dm3/2 mol — 1/2 s — 1/2](2.53 ± 0.60) — (39.5 ± 2.4) kJ mol — 1/θ 其中 θ=RT In 10. 对于部首 2-ä3H7 和 (Z)-ä4H7 的交叉组合比, Ï[2-ä3H7, (Z)-ä得出4H7]t= 2.12 ± 0.10,其中下标t表示末端组合。在各种加成/异构化/解离过程中观察到某些特征产物的形成。建议将 2-ä3H7 添加到 (E)- CHCH 作为速率决定步骤,然后进行 1、4- 和 1,5-H 原子位移。
-
Factors Influencing the Direction of Elimination in Ester Pyrolyses作者:Robert A. Benkeser、James J. Hazdra、Merwyn L. BurrousDOI:10.1021/ja01529a031日期:1959.10
-
Kasanskii et al., Doklady Akademii Nauk SSSR, 1955, vol. 105, p. 485,487作者:Kasanskii et al.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
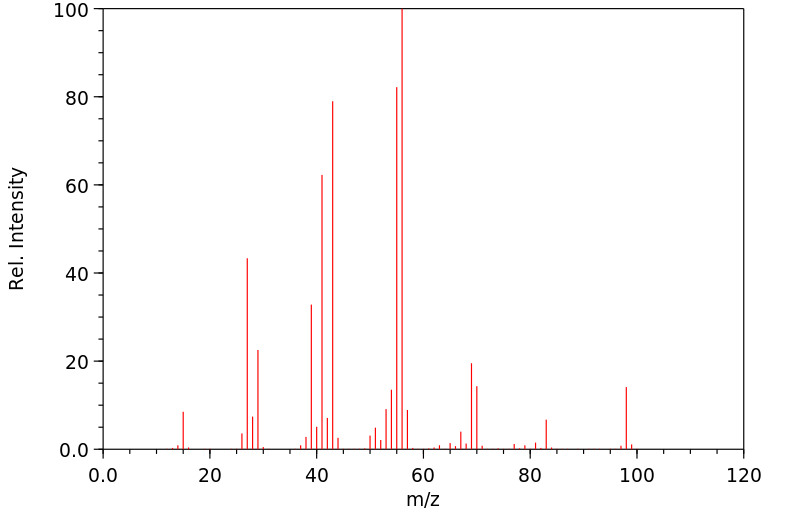
质谱MS
-
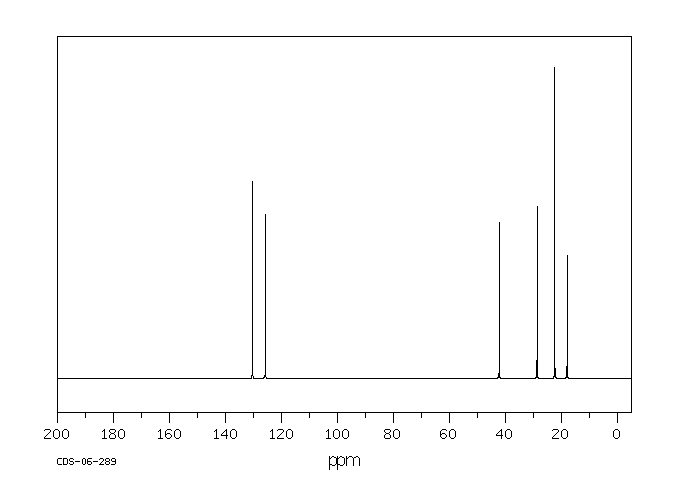
碳谱13CNMR
-
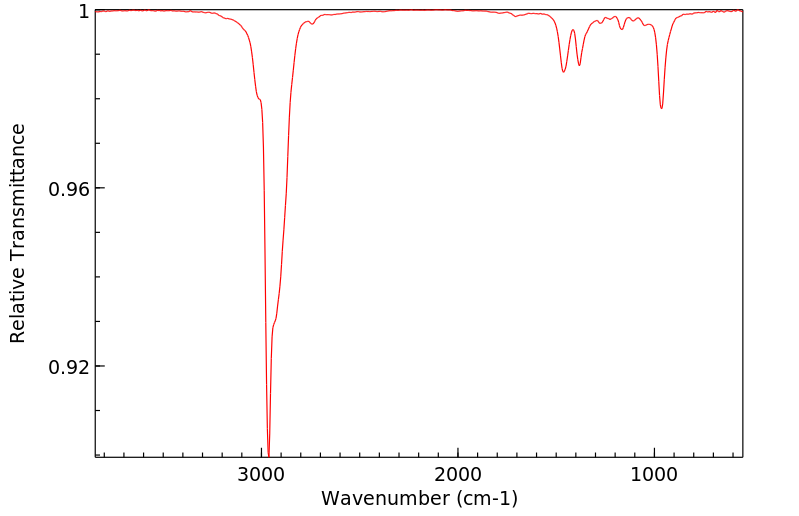
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-