(E)-2-methyl-1,5-heptadien-4-ol | 107791-76-4
中文名称
——
中文别名
——
英文名称
(E)-2-methyl-1,5-heptadien-4-ol
英文别名
1,5-dimethyl-1,5-hexadiene-3-ol;2-Methyl-1,5-heptadien-4-ol;2-methyl-hepta-1,5t-dien-4-ol;2-Methyl-hepta-1,5-dien-4-ol;(5E)-2-methylhepta-1,5-dien-4-ol
CAS
107791-76-4
化学式
C8H14O
mdl
——
分子量
126.199
InChiKey
MDTLRZIDFDKYHS-SNAWJCMRSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:185.3±9.0 °C(Predicted)
-
密度:0.858±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:9
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (+)-(R)-2-methyl-1,5-heptadien-4-ol 153254-11-6 C8H14O 126.199
反应信息
-
作为反应物:描述:参考文献:名称:Vapor-phase thermolyses of 3-hydroxy-1,5-hexadienes. II. Effects of methyl substitution摘要:DOI:10.1021/ja00990a019
-
作为产物:描述:6-chloro-2-methyl-4,5-epoxy-1-heptene 在 magnesium 作用下, 以 四氢呋喃 为溶剂, 以74%的产率得到(E)-2-methyl-1,5-heptadien-4-ol参考文献:名称:摘要:Substituted 1,5-hexadien-3-ols were synthesized by the [2,3]-Wittig rearrangement of unsymmetrical bis-allyl ethers, as well as by reactions of 1-(2-alkenyl)-2-chloromethyloxiranes with Mg/THF. The products were oxidized with pyridinium chlorochromate (PCC), zinc chlorochromate (ZCC), tert-butyl hydroperoxide in the presence of OsO4, and tent-butyl hydroperoxide alone. The oxidation of substituted 1,5-hexadien-3-ols with PCC and ZCC gave the corresponding carbonyl compounds. In the reaction with tertbutyl hydroperoxide catalyzed by OsO4 the internal double bond in the substrate was regioselectively converted into epoxy group, whereas allylic oxidation was prevented.DOI:10.1023/a:1013843700623
文献信息
-
An Investigation of the Reactions of Substituted Homoallylic Alcohols with Various Oxidation Reagents作者:S. Servi、A. AcarDOI:10.3390/70200104日期:——Substituted homoallylic alcohols have been synthesised both by [2,3]-Wittig rearrangement of unsymmetrical bis-allylic ethers and reaction of alkenyl chloromethyl oxiranes with Mg/THF. These substrates were then oxidized using four different oxidants. When the substituted homoallylic alcohols were oxidized with pyridinium chlorochromate or zinc chlorochromate nonahydrate the corresponding carbonyl
-
Selective epoxidation of allylic alcohols with dibutyltin oxyperoxide
-
Oxyanion Orientation in Anionic Oxy-Cope Rearrangements作者:Eun Lee、Yong Rok Lee、Bongjin Moon、Ohyun Kwon、Mi Seong Shim、Jae Sook YunDOI:10.1021/jo00085a037日期:1994.3Efficiency of chirality transfer in anionic oxy-Cope rearrangement depends solely on the orientational preference of the oxyanionic bond in the substrates with a single carbinol carbon chiral center. In chairlike transition-state conformations for the rearrangement of simplest substrates like anions generated from (E)-1-phenyl-1,5-hexadien-3-ol and (E)-1,5-heptadien-4-ol, the oxyanionic bond is more prone to adopt the pseudoaxial orientation. On the other hand, anions generated from (E)-2-methyl-1,5-heptadien-4-ol, (E)-5-tert-butyl-1-phenyl-1,5-hexadien-3-ol, and 4-(2'-methyl-1'-cyclohexenyl)-1-buten-3-ol undergo rearrangement via chairlike transition states in which the pseudoequatorial oxyanionic bond is favored. It can thus be surmized that there is a slight stereoelectronic preference for the pseudoaxial oxyanionic bond in the chairlike transition states for the rearrangement of substrates without steric constraints. Substitution at C5 of the basic 1,5-hexadien-3-ol framework of substrates, however, leads to 1,3-diaxial steric interaction in the chairlike transition states with pseudoaxial oxyanionic bond, and pseudoequatorial disposition of oxyanionic bond becomes more favorable.
-
Asymmetric Synthesis of 2°- and 3°-Carbinols via <i>B</i>-Methallyl-10-(TMS and Ph)-9-borabicyclo[3.3.2]decanes作者:José G. Román、John A. SoderquistDOI:10.1021/jo701633k日期:2007.12.1[GRAPHICS]Simple Grignard procedures provide methallylboranes la and 1b in enantiomerically pure form from air-stable precursors in 98% and 95% yields, respectively. These reagents add smoothly to aldehydes and methyl ketones, respectively, providing branched 2 degrees- (6, 69-89%, 94-99% ee) and 3 degrees-(10, 71-87%, 74-96% ee) homoallylic alcohols.
-
Use of Hine's D Values To Predict the Position of the Equilibrium in the Cope Rearrangement of Multiply Substituted 1,5-Dienes作者:James P. Hagen、Kemberly D. Lewis、Scott W. Lovell、Paolo Rossi、Ayse Z. TezcanDOI:10.1021/jo00128a019日期:1995.11A series of 1,5-dienes (1a-f) were employed to test whether Hine's D values can predict the position of equilibrium in Cope rearrangements. In the cases of the substituent pairs [OCH3, H], [OCH3, CH3], [N(CH3)(2), H], [N(CH3)(2), CH3], and [N(CH3)(2), OCH3], equilibrium constants calculated with Hine's D values gave reasonable agreement with those obtained experimentally. Dienes 1g-i were prepared to test whether reduction of the pi-donating character of a nitrogen substituent (carbamoyl vs dimethylamino) would change the directing ability of the nitrogen group. The aggregate order of directing ability was N(CH3)(2) > OCH3 > EtO(2)CN(CH3) > CH3 > A. Diene 15a, with a more complicated substitution pattern (OCH3 and CH3 versus CH3 and H) not directly amenable to analysis with D values, can be considered to reduce to the case of [OCH3, H]. The experimental K-eq obtained agreed with that expected for the [OCH3, H] pair. Dienes 15c and 16b, designed to test the pairs [CH3, SPh] and [OCH3, SPh], respectively, decomposed under the gas phase conditions of the rearrangement. Attempts to effect rearrangement with Pd(II) catalysis failed.
表征谱图
-
氢谱1HNMR
-
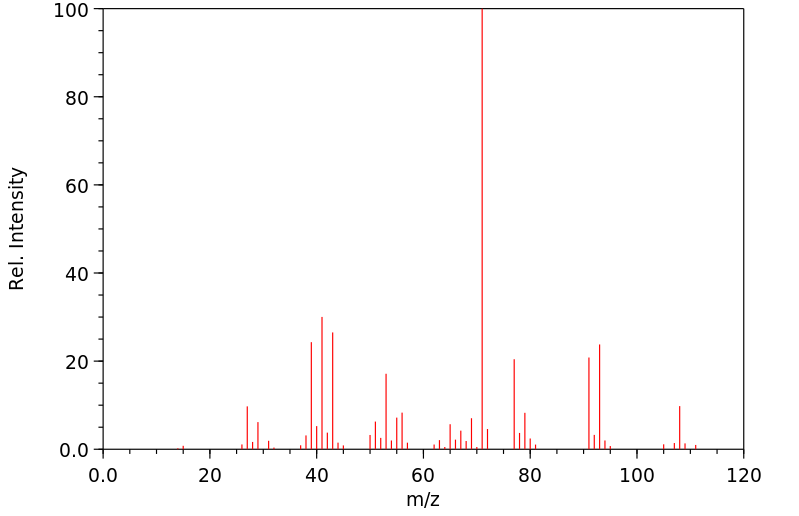
质谱MS
-
碳谱13CNMR
-
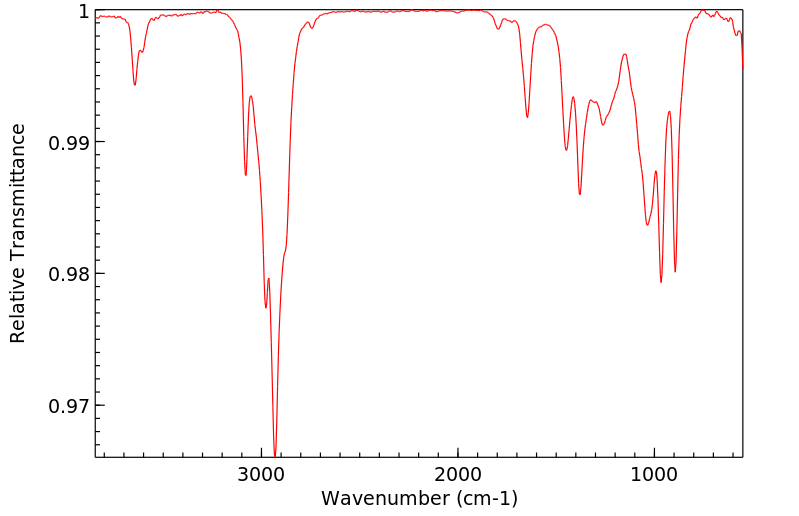
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷