5-甲基-1,5-己二烯-3-醇 | 17123-61-4
中文名称
5-甲基-1,5-己二烯-3-醇
中文别名
——
英文名称
5-methyl-1,5-hexadiene-3-ol
英文别名
5-methyl-1,5-hexadien-3-ol;5-methyl-hexa-1,5-dien-3-ol;5-methylhexa-1,5-dien-3-ol
CAS
17123-61-4
化学式
C7H12O
mdl
MFCD00048134
分子量
112.172
InChiKey
AVMGRIXBDWUWFN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:62-63 °C(Press: 23 Torr)
-
密度:0.8612 g/cm3
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:8
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.428
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2905290000
SDS
反应信息
-
作为反应物:描述:5-甲基-1,5-己二烯-3-醇 在 chromium(VI) oxide 、 硫酸 作用下, 以 甲醇 、 乙醚 、 水 为溶剂, 生成 4-Methylbicyclo<2.1.1>hexan-2-on参考文献:名称:1,5-己二烯-3-酮的光化学:分子内烯酮-烯烃光加成的波长依赖性选择性摘要:通过使用单色光,在 313-366 nm 的波长范围内研究了十个 1,5-己二烯-3-酮在甲醇中的光化学。为了了解对 5-methyl-1,5-hexadien-3-one (1) 观察到的不寻常的波长依赖性选择性,进行了量子产率测量、结构反应性研究、三线态敏化和淬火实验。虽然 10 项二烯酮研究中有 6 项显示选择性与 313 至 366 nm 的辐射波长无关,但有 4 项二烯酮表现出与波长相关的选择性,与 1 中观察到的相似,因此确定 1 不是唯一的,以前认为. 三线态敏化研究表明,波长依赖性主要来自以三线态环加成完成的单态α-裂解反应。多种三线态猝灭剂在抑制这些反应方面是无效的。讨论了一些可能的机制DOI:10.1021/ja00015a041
-
作为产物:描述:参考文献:名称:摘要:Substituted 1,5-hexadien-3-ols were synthesized by the [2,3]-Wittig rearrangement of unsymmetrical bis-allyl ethers, as well as by reactions of 1-(2-alkenyl)-2-chloromethyloxiranes with Mg/THF. The products were oxidized with pyridinium chlorochromate (PCC), zinc chlorochromate (ZCC), tert-butyl hydroperoxide in the presence of OsO4, and tent-butyl hydroperoxide alone. The oxidation of substituted 1,5-hexadien-3-ols with PCC and ZCC gave the corresponding carbonyl compounds. In the reaction with tertbutyl hydroperoxide catalyzed by OsO4 the internal double bond in the substrate was regioselectively converted into epoxy group, whereas allylic oxidation was prevented.DOI:10.1023/a:1013843700623
文献信息
-
Transposition oxy-cope assistee par le trifluoroacetate mercurique en quantite stoechiometrique et en quantite catalytique作者:Norbert Bluthe、Max Malacria、Jacques GoreDOI:10.1016/0040-4020(84)85011-5日期:1984.1Tertiary 1,5-hexadien-3-ols are transformed at room temperature into δ-ethylenic ketones in 35-90%, yields under two sets of conditions: treatment with one molar equivalent of mercuric trifluoroacetate followed by demercuration of the intermediate α-mercuro ketone with sodium borohydride; and treatment with 0.2 molar equivalent of t of lithium trifluoroacetate or trifluorométhansulfonate. The reactions
-
Catalytic pyrolysis of cellulose in ionic liquid [bmim]OTf作者:Guangfei Qu、Weiwei He、Yingying Cai、Xi Huang、Ping NingDOI:10.1016/j.carbpol.2016.04.052日期:2016.9This study discussed the catalytic cracking process of cellulose in ionic liquid 1-butyl-3-methylimidazolium trifluoromethanesulfonate ([bmim]OTF) under 180°C, 240°C and 340°C, found that [bmim]OTF is an effective catalyst which can effectively reduce the pyrolysis temperature(nearly 200°C) of the cellulose. FRIR, XRD and SEM were used to analyze the structure characterization of fiber before and after
-
An Investigation of the Reactions of Substituted Homoallylic Alcohols with Various Oxidation Reagents作者:S. Servi、A. AcarDOI:10.3390/70200104日期:——Substituted homoallylic alcohols have been synthesised both by [2,3]-Wittig rearrangement of unsymmetrical bis-allylic ethers and reaction of alkenyl chloromethyl oxiranes with Mg/THF. These substrates were then oxidized using four different oxidants. When the substituted homoallylic alcohols were oxidized with pyridinium chlorochromate or zinc chlorochromate nonahydrate the corresponding carbonyl
-
Vapor-phase thermolyses of 3-hydroxy-1,5-hexadienes. II. Effects of methyl substitution作者:Alfred. Viola、E. James. Iorio、Katherine K. N. Chen、George M. Glover、Ullhas. Nayak、Philip J. KocienskiDOI:10.1021/ja00990a019日期:1967.7
-
Photochemistry of substituted 1,5-hexadien-3-ones作者:Thomas W. Gibson、William F. ErmanDOI:10.1021/jo00973a017日期:1972.4
表征谱图
-
氢谱1HNMR
-
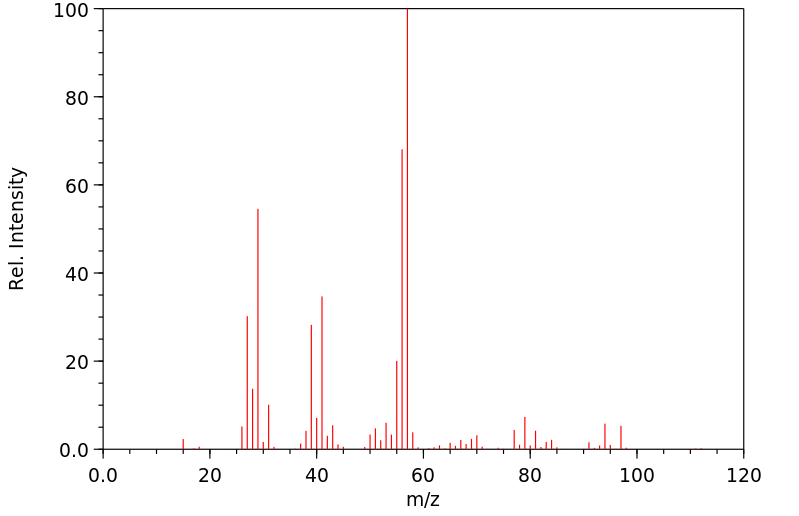
质谱MS
-
碳谱13CNMR
-
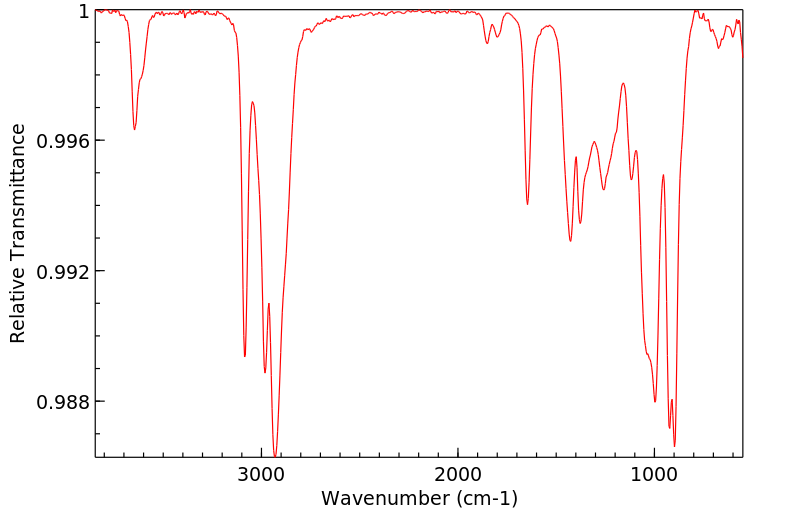
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷