5-羟基-2-甲基吲哚-3-羧酸乙酯 | 7598-91-6
中文名称
5-羟基-2-甲基吲哚-3-羧酸乙酯
中文别名
5-羟基-2-甲基吲哚-3-甲酸乙酯;5-羟基-2-甲基-1H-吲哚-3-甲酸乙酯;2-甲基-(3-甲酸乙酯)-5-羟基吲哚
英文名称
ethyl 5-hydroxy-2-methylindole-3-carboxylate
英文别名
ethyl 5-hydroxy-2-methyl-1H-indole-3-carboxylate
CAS
7598-91-6
化学式
C12H13NO3
mdl
MFCD00044575
分子量
219.24
InChiKey
BSNHKSUAAMJXBB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:205-208 °C (lit.)
-
沸点:360.05°C (rough estimate)
-
密度:1.1825 (rough estimate)
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:16
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:62.3
-
氢给体数:2
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
海关编码:2933990090
-
安全说明:S22,S24/25
-
危险性防范说明:P261,P301+P312,P302+P352,P304+P340,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:应密封保存在阴凉干燥的环境中。
SDS
模块 1. 化学品
1.1 产品标识符
: 5-羟基-2-甲基吲哚-3-羧酸乙酯
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C12H13NO3
分子式
: 219.24 g/mol
分子量
无
模块 4. 急救措施
4.1 必要的急救措施描述
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。
皮肤接触
用肥皂和大量的水冲洗。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。
4.2 主要症状和影响,急性和迟发效应
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
避免粉尘生成。 避免吸入蒸气、烟雾或气体。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
个体防护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所选择身体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 205 - 208 °C - lit.
f) 沸点、初沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— propyl 5-hydroxy-2-methyl-1H-indole-3-carboxylate 27363-10-6 C13H15NO3 233.267 —— 2-Methyl-3-isopropyloxycarbonyl-5-hydroxy-indol 54436-15-6 C13H15NO3 233.267 5-甲氧基-2-甲基吲哚-3-羧酸乙酯 ethyl 5-methoxy-2-methyl-1H-indole-3-carboxylate 34572-31-1 C13H15NO3 233.267 5-乙酰氧基-2-甲基吲哚-3-羧酸乙酯 ethyl 5-acetyloxy-2-methyl-1H-indole-3-carboxylate 20862-91-3 C14H15NO4 261.277 —— 5-benzyloxy-2-methyl-indole-3-carboxylic acid ethyl ester 101890-42-0 C19H19NO3 309.365 6-溴-5-羟基2-甲基-1H-吲哚-3-羧酸乙酯 ethyl 6-bromo-5-hydroxy-2-methyl-indole-3-carboxylate 16052-67-8 C12H12BrNO3 298.136 5-羟基-2-甲基-1H-吲哚-3-羧酸 5-hydroxy-2-methyl-1H-indole-3-carboxylic acid 71982-15-5 C10H9NO3 191.186 5-甲氧基-2-甲基-1H-吲哚-3-羧酸 5-methoxy-2methyl-1H-indole-3-carboxylic acid 32387-22-7 C11H11NO3 205.213 美卡比酯 mecarbinate 15574-49-9 C13H15NO3 233.267 5-甲氧基-1,2-二甲基吲哚-3-羧酸乙酯 ethyl 5-methoxy-1,2-dimethyl-1H-indole-3-carboxylate 40963-98-2 C14H17NO3 247.294 5-乙酰氧基-1,2-二甲基吲哚-3-羧酸乙酯 ethyl 5-acetoxy-1,2-dimethylindole-3-carboxylate 40945-79-7 C15H17NO4 275.304 —— 4-dimethylaminomethyl-5-hydroxy-2-methyl-indole-3-carboxylic acid ethyl ester 13098-13-0 C15H20N2O3 276.335 —— propyl 4-[(dimethylamino)methyl]-5-hydroxy-2-methyl-1H-indole-3-carboxylate 283607-96-5 C16H22N2O3 290.362 —— 2-Methyl-3-ethoxycarbonyl-4,6-bis(dimethylaminomethyl)-5-hydroxyindole 88461-89-6 C18H27N3O3 333.431 5-羟基-2-甲基-1-苯基-1H-吲哚-3-羧酸乙酯 1-phenyl-2-methyl-3-ethoxycarbonyl-5-hydroxyindole 5564-29-4 C18H17NO3 295.338 5-羟基-2-甲基-1-对甲苯基-1H-吲哚-3-羧酸乙酯 ethyl 5-hydroxy-2-methyl-1-(p-tolyl)-1H-indole-3-carboxylate 5165-45-7 C19H19NO3 309.365 —— ethyl 5-hydroxy-1-methyl-2-(phenylthiomethyl)-1H-indole-3-carboxylate 26065-16-7 C19H19NO3S 341.431 —— ethyl 2-((4-fluorophenylthio)methyl)-5-hydroxy-1-methyl-1H-indole-3-carboxylate 1615239-33-2 C19H18FNO3S 359.421 —— ethyl 5-hydroxy-2-((4-methoxyphenylthio)methyl)-1-methyl-1H-indole-3-carboxylate 1615239-29-6 C20H21NO4S 371.457 —— ethyl 2-((3-chlorophenylthio)methyl)-5-hydroxy-1-methyl-1H-indole-3-carboxylate 1615239-34-3 C19H18ClNO3S 375.876 —— ethyl 2-((3,5-dimethylphenylthio)methyl)-5-hydroxy-1-methyl-1H-indole-3-carboxylate 1615239-38-7 C21H23NO3S 369.485 —— ethyl 5-hydroxy-1-methyl-2-((4-(trifluoromethyl)phenylthio)methyl)-1H-indole-3-carboxylate 1615239-32-1 C20H18F3NO3S 409.429 5-乙酰氧基-6-溴-2-溴甲基-1-甲基吲哚-3-甲酸乙酯 1-methyl-2-bromomethyl-3-ethoxycarbonyl-5-acetoxy-6-bromoindole 110543-98-1 C15H15Br2NO4 433.096 —— ethyl 2-((2,6-dimethylphenylthio)methyl)-5-hydroxy-1-methyl-1H-indole-3-carboxylate 1615239-37-6 C21H23NO3S 369.485 —— ethyl 5-hydroxy-1-methyl-2-((4-(trifluoromethoxy)phenylthio)methyl)-1H-indole-3-carboxylate 1615239-30-9 C20H18F3NO4S 425.428 —— ethyl 2-((2,4-difluorophenylthio)methyl)-5-hydroxy-1-methyl-1H-indole-3-carboxylate 1615239-35-4 C19H17F2NO3S 377.412 —— ethyl 5-hydroxy-2-(mesitylthiomethyl)-1-methyl-1H-indole-3-carboxylate 1615239-39-8 C22H25NO3S 383.511 —— ethyl 2-((2,6-dichlorophenylthio)methyl)-5-hydroxy-1-methyl-1H-indole-3-carboxylate 1615239-36-5 C19H17Cl2NO3S 410.321 6-溴-5-羟基-1-甲基-2-(苯基硫甲基)吲哚-3-甲酸乙酯 ethyl 6-bromo-5-hydroxy-1-methyl-2-(phenylthiomethyl)indole-3-carboxylate 131707-24-9 C19H18BrNO3S 420.327 —— ethyl 5-hydroxy-2-((2-methoxyphenylthio)methyl)-1-methyl-1H-indole-3-carboxylate 1615239-28-5 C20H21NO4S 371.457 —— ethyl 5-hydroxy-1-methyl-2-((2-(trifluoromethyl)phenylthio)methyl)-1H-indole-3-carboxylate 1615239-31-0 C20H18F3NO3S 409.429 —— N-Formyl-5-hydroxy-2-methylindole-3-carboxyhydrazide 77294-35-0 C11H11N3O3 233.227 —— N-Acetyl-5-hydroxy-2-methylindole-3-carboxyhydrazide 77294-36-1 C12H13N3O3 247.254 9-甲氧基-2-甲基-3,11,12,14-四氢-6aH-吲哚并[4',5':5,6][1,3]恶嗪并[2,3-a]异喹啉-1-羧酸乙酯 PD 102807 23062-91-1 C23H24N2O4 392.455 阿比朵尔 arbidol 131707-25-0 C22H25BrN2O3S 477.422 —— 3-benzyl-2-methyl-1H-indole-5-ol 440351-67-7 C16H15NO 237.301 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:描述:5-羟基-2-甲基吲哚-3-羧酸乙酯 在 吡啶 、 溴 、 sodium hydride 、 potassium hydroxide 作用下, 以 1,4-二氧六环 、 甲醇 、 四氯化碳 、 N,N-二甲基甲酰胺 为溶剂, 反应 25.25h, 生成 阿比朵尔参考文献:名称:与流感血凝素亲和力明显提高的抗病毒药物Arbidol类似物的基于结构的优化和合成摘要:流感是一种高度传染性的呼吸道病毒感染,仅在美国每年就造成50,000例死亡。对具有新颖作用方式的新疗法的需求至关重要。我们确定了带有流感血凝素的Arbidol的X射线结构,发现它位于明显的结合口袋中。在本文中,我们报告了基于共配合物与生物层干涉法相结合的结构-活性关系研究,以评估我们化合物的结合。加元-Arbidol的硫酚部分的羟基取代了结合口袋中的结构化水分子,导致对H3(1150倍)和H1(98倍)血凝素亚型的亲和力显着增加。我们的类似物代表了新颖的线索,可产生更强大的抗血凝素化合物,从而阻止病毒进入。DOI:10.1016/j.bmcl.2017.06.074
-
作为产物:描述:4-氨基苯基三氟硼酸钾 在 indium(III) chloride 、 N-溴代丁二酰亚胺(NBS) 、 双氧水 、 (1R,2R)-(-)-N,N'-二甲基-1,2-环己二胺 、 copper(I) bromide 作用下, 以 乙醇 、 水 、 乙酸乙酯 为溶剂, 反应 17.5h, 生成 5-羟基-2-甲基吲哚-3-羧酸乙酯参考文献:名称:一种阿比朵尔关键中间体的制备方法摘要:本发明公开了一种阿比朵尔关键中间体的制备方法,属于药物合成技术领域。4‑氨基苯硼酸衍生物1与NBS溴代反应得到中间体2,接着在铟催化剂作用下与乙酰乙酸乙酯缩合得到中间体3,随后铜盐催化关环反应得到中间体4,最后通过双氧水氧化得到5‑羟基‑2‑甲基‑1H‑吲哚‑3‑羧酸乙酯5。本发明工艺操作简便稳定,产率高,环境友好,较现有的工艺,避免了毒害大、易升化的苯醌,大幅降低了现有阿比朵尔中间体的生产安全风险,产品纯度高达99.5%,单杂低于0.1%。公开号:CN112694432B
文献信息
-
α-d-Galacturonic Acid as Natural Ligand for Selective Copper-Catalyzed N-Arylation of N-Containing Heterocycles作者:Chunling Yuan、Yingdai Zhao、Li ZhengDOI:10.1055/s-0039-1690226日期:2019.12
The first application of α-d-galacturonic acid hydrate (GalA) is reported here, as a potential O-donor ligand. The C–N couplings of N-heterocycles with aryl halides or arylboronic acids could be readily conducted and exhibited good functional group tolerance with characters of selectivity. These N-arylazoles allow rapid access to several pharmaceutical intermediates and can be easily transformed into a variety of other interesting scaffolds as well.
-
Indolequinone Antitumor Agents: Reductive Activation and Elimination from (5-Methoxy-1-methyl-4,7-dioxoindol-3-yl)methyl Derivatives and Hypoxia-Selective Cytotoxicity in Vitro作者:Matthew A. Naylor、Elizabeth Swann、Steven A. Everett、Mohammed Jaffar、John Nolan、Naomi Robertson、Stacey D. Lockyer、Kantilal B. Patel、Madeleine F. Dennis、Michael R. L. Stratford、Peter Wardman、Gerald E. Adams、Christopher J. Moody、Ian J. StratfordDOI:10.1021/jm970744w日期:1998.7.1of the 3-hydroxymethyl derivative, radiolytic generation of semiquinone radicals and HPLC analysis indicated that efficient elimination of the leaving group occurred following one-electron reduction of the parent compound. The active species in leaving group elimination was predominantly the hydroquinone rather than the semiquinone radical. The resulting iminium derivative acted as an alkylating agent通过相应的3-(羟甲基)吲哚醌的功能化合成了一系列在(indol-3-yl)甲基位置具有多种离去基团的吲哚醌,并将所得化合物作为生物还原活化的细胞毒素进行体外评估。在化学和定量辐解条件下还原活化后,证明了一系列官能团(羧酸盐、苯酚和硫醇)的消除。只有在两组条件下消除这些基团的化合物才表现出显着的缺氧选择性,缺氧:好氧毒性比在 10-200 范围内。除 3-羟甲基衍生物外,半醌自由基的辐射分解产生和高效液相色谱分析表明,离去基团的有效消除发生在母体化合物的单电子还原之后。离去基团消除的活性物质主要是氢醌而不是半醌自由基。得到的亚胺衍生物作为烷基化剂,在化学还原后被添加的硫醇有效地捕获,在辐射还原后被水或 2-丙醇有效地捕获。观察到在氢供体(2-丙醇)存在下自由基引发的这些吲哚醌(在更简单的苯醌中未见)还原的链式反应。在 V79-379A 细胞中,发现 C-2 处未取代的化合物作为细胞毒素的效力是其
-
Methods for forming combinatorial libraries combining amide bond formation with epoxide opening申请人:——公开号:US20020077491A1公开(公告)日:2002-06-20The invention relates to methods for forming combinatorial libraries. The invention provides methods suitable for the rapid and convenient synthesis of very large combinatorial libraries of small organic molecules. In particular, the invention provides a method for forming combinatorial libraries combining amide bond formation with epoxide opening.这项发明涉及形成组合库的方法。该发明提供了适用于快速和便捷合成非常大的小有机分子组合库的方法。具体而言,该发明提供了一种将酰胺键形成与环氧化物开启相结合的组合库形成方法。
-
[EN] ARBIDOL ANALOGS WITH IMPROVED INFLUENZA HEMAGGLUTININ POTENCY<br/>[FR] ANALOGUES D'ARBIDOL AYANT UNE PUISSANCE AMÉLIORÉE VIS-À-VIS DE L'HÉMAGGLUTININE D'INFLUENZA申请人:SCRIPPS RESEARCH INST公开号:WO2018112128A1公开(公告)日:2018-06-21The invention provides a series of analogs of arbidol having enhanced binding activity with respect to influenza hemagglutinin. Accordingly, the invention can provide a method of inhibiting the bioactivity of viral hemagglutinin activity, which is an essential step in the entry of infectious viral particles into host cells. The invention also can provide a method of treatment of influence, comprising administering an effective amount of a compound of formula (A), wherein X is S or O, to a patient afflicted therewith.这项发明提供了一系列与阿比多尔类似物,其在与流感血凝素结合活性方面具有增强的效果。因此,该发明可以提供一种抑制病毒血凝素生物活性的方法,这是感染性病毒颗粒进入宿主细胞的关键步骤。该发明还可以提供一种治疗流感的方法,包括向患者施用化合物(A)的有效剂量,其中X为S或O。
-
Synthesis and Pharmacology of Benzoxazines as Highly Selective Antagonists at M<sub>4</sub> Muscarinic Receptors作者:Thomas M. Böhme、Corinne E. Augelli-Szafran、Hussein Hallak、Thomas Pugsley、Kevin Serpa、Roy D. SchwarzDOI:10.1021/jm011116o日期:2002.7.1102807 (41) as being the most selective synthetic M(4) muscarinic antagonist identified to date. Synthesized analogues of 41 showed no improvement in affinity and selectivity at that time. However, several newly synthesized compounds exhibit a 7-fold higher affinity at M(4) receptors and demonstrate a selectivity of at least 100-fold over all other muscarinic receptor subtypes. For example, compound以前,我们在PD 102807(41)上报道其为迄今为止鉴定出的最具选择性的合成M(4)毒蕈碱拮抗剂。当时合成的41类似物没有显示亲和力和选择性的改善。但是,几种新合成的化合物在M(4)受体上显示出7倍更高的亲和力,并且比所有其他毒蕈碱受体亚型表现出至少100倍的选择性。例如,化合物28对M(4)受体显示pK(i)= 9.00的亲和力,对M(1)/ M(4)= 13183-fold,M(2)/ M(4)= 339倍,M(3)/ M(4)= 151倍和M(5)/ M(4)= 11220倍。尚未针对任何合成毒蕈碱拮抗剂或天然M(4)拮抗剂如M(4)选择性东部绿曼巴蛇毒MT3(M(4)pK(b)= 8.7)报道过这种高选择性和高亲和力。 ,M(1)/ M(4)= 40倍,M(2)/ M(4)>或= 500倍,M(3)/ M(4)>或= 500倍,以及M(5)/ M(4)>或= 500倍)。衍生物
表征谱图
-
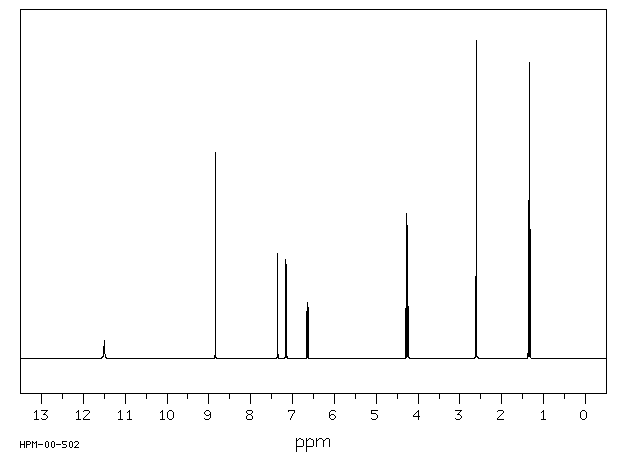
氢谱1HNMR
-
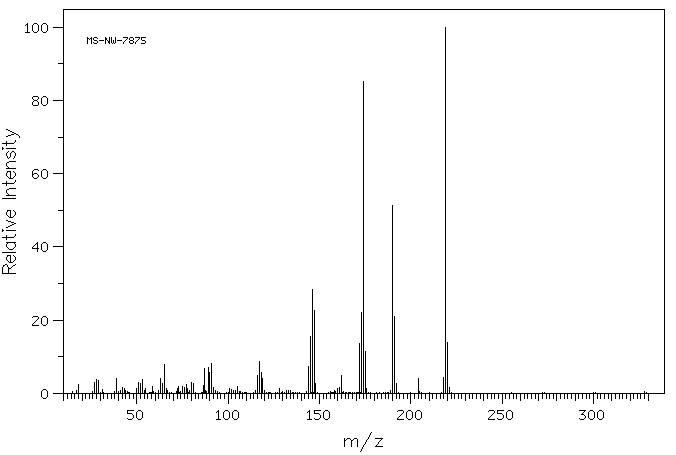
质谱MS
-
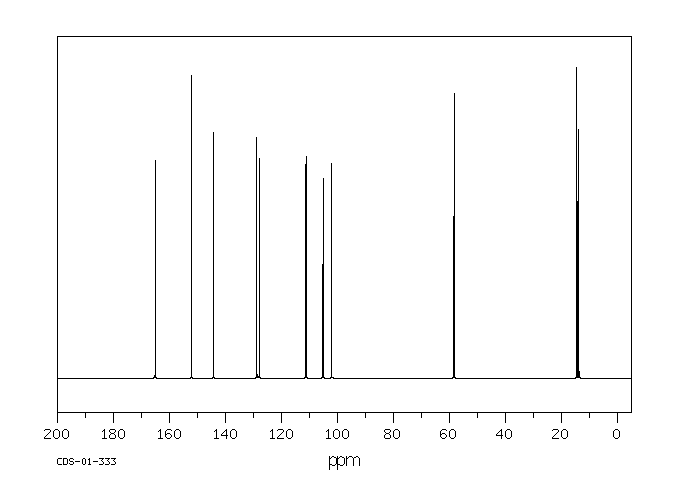
碳谱13CNMR
-
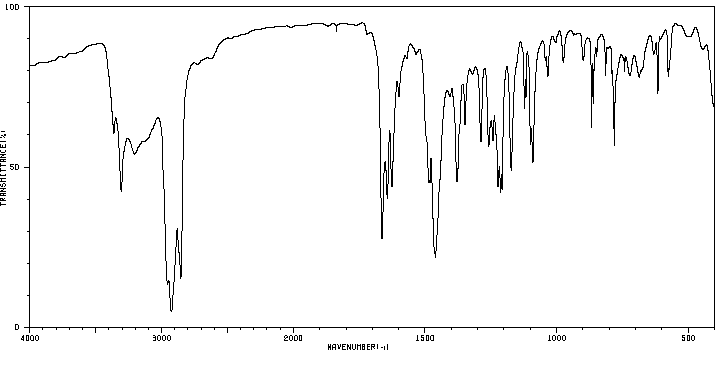
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Z)-3-[[[2,4-二甲基-3-(乙氧羰基)吡咯-5-基]亚甲基]吲哚-2--2-
(S)-(-)-5'-苄氧基苯基卡维地洛
(R)-(+)-5'-苄氧基卡维地洛
(R)-卡洛芬
(N-(Boc)-2-吲哚基)二甲基硅烷醇钠
(E)-2-氰基-3-(5-(2-辛基-7-(4-(对甲苯基)-1,2,3,3a,4,8b-六氢环戊[b]吲哚-7-基)-2H-苯并[d][1,2,3]三唑-4-基)噻吩-2-基)丙烯酸
(4aS,9bR)-6-溴-2,3,4,4a,5,9b-六氢-1H-吡啶并[4,3-B]吲哚
(3Z)-3-(1H-咪唑-5-基亚甲基)-5-甲氧基-1H-吲哚-2-酮
(3Z)-3-[[[4-(二甲基氨基)苯基]亚甲基]-1H-吲哚-2-酮
(3R)-(-)-3-(1-甲基吲哚-3-基)丁酸甲酯
(3-氯-4,5-二氢-1,2-恶唑-5-基)(1,3-二氧代-1,3-二氢-2H-异吲哚-2-基)乙酸
齐多美辛
鸭脚树叶碱
鸭脚木碱,鸡骨常山碱
鲜麦得新糖
高氯酸1,1’-二(十六烷基)-3,3,3’,3’-四甲基吲哚碳菁
马鲁司特
马鞭草(VERBENAOFFICINALIS)提取物
马来酸阿洛司琼
马来酸替加色罗
顺式-ent-他达拉非
顺式-1,3,4,4a,5,9b-六氢-2H-吡啶并[4,3-b]吲哚-2-甲酸乙酯
顺式-(+-)-3,4-二氢-8-氯-4'-甲基-4-(甲基氨基)-螺(苯并(cd)吲哚-5(1H),2'(5'H)-呋喃)-5'-酮
靛青二磺酸二钾盐
靛藍四磺酸
靛红联二甲酚
靛红磺酸钠
靛红磺酸
靛红乙烯硫代缩酮
靛红-7-甲酸甲酯
靛红-5-磺酸钠
靛红-5-磺酸
靛红-5-硫酸钠盐二水
靛红-5-甲酸甲酯
靛红
靛玉红衍生物E804
靛玉红3'-单肟5-磺酸
靛玉红-3'-单肟
靛玉红
靛噻
青色素3联己酸染料,钾盐
雷马曲班
雷莫司琼杂质13
雷莫司琼杂质12
雷莫司琼杂质
雷替尼卜定
雄甾-1,4-二烯-3,17-二酮
阿霉素的代谢产物盐酸盐
阿贝卡尔
阿西美辛杂质3