5-降冰片烯-2,3-二羧酸 | 3853-88-1
中文名称
5-降冰片烯-2,3-二羧酸
中文别名
顺-5-降冰片烯-endo-2,3-二羧酸;顺-5-降冰片烯-内-2,3-二羧酸;双环[2.2.1]庚-5-烯-2,3-二羧酸
英文名称
cis-5-norbornene-endo-2,3-dicarboxylic acid
英文别名
endo-norbornene-cis-5,6-dicarboxylic acid;endic acid;(1S,2R,3S,4R)-bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic acid
CAS
3853-88-1
化学式
C9H10O4
mdl
——
分子量
182.176
InChiKey
NIDNOXCRFUCAKQ-UMRXKNAASA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:175 °C (dec.)(lit.)
-
沸点:419.8±45.0 °C(Predicted)
-
密度:1.481±0.06 g/cm3(Predicted)
-
稳定性/保质期:
避免使用强氧化剂。
计算性质
-
辛醇/水分配系数(LogP):0.3
-
重原子数:13
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.56
-
拓扑面积:74.6
-
氢给体数:2
-
氢受体数:4
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xn
-
安全说明:S26,S37/39
-
危险类别码:R20/21/22,R36/37/38
-
WGK Germany:3
-
海关编码:2917209090
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302+H312+H332,H315,H319,H335
-
储存条件:存储在密封容器中,并阴凉干燥处保存。
SDS
| Name: | Bicyclo[2.2.1]hept-5-ene-2 3-dicarboxylic acid 97% Material Safety Data Sheet |
| Synonym: | exo, exo Bicyclo[2.2.1]hept-2-ene-5,6-dicarboxylic aci |
| CAS: | 3853-88-1 |
Synonym:exo, exo Bicyclo[2.2.1]hept-2-ene-5,6-dicarboxylic aci
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 3853-88-1 | Bicyclo[2.2.1]hept-5-ene-2,3-dicarboxy | 97% | unlisted |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 3853-88-1: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 145 - 147 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C9H10O4
Molecular Weight: 182.17
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 3853-88-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic acid - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 3853-88-1: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 3853-88-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 3853-88-1 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— endo-bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic dimethyl ester 39589-98-5 C11H14O4 210.23 —— 3,6-endomethylene-1,2,3,6-tetrahydrophthalic anhydride 129-64-6 C9H8O3 164.161 —— 5-norbornene-2,3-dicarboxylic anhydride 129-64-6 C9H8O3 164.161 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 降冰片烯-2,3-二羧酸 norbornene-5,6-dicarboxylic acid 1200-88-0 C9H10O4 182.176 —— endo-bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic dimethyl ester 39589-98-5 C11H14O4 210.23 —— diethyl (endo,endo)-bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylate 7288-32-6 C13H18O4 238.284 —— dipent-3-ynyl endo,cis-norborn-5-ene-2,3-dicarboxylate 951697-39-5 C19H22O4 314.381 —— 3,6-endomethylene-1,2,3,6-tetrahydrophthalic anhydride 129-64-6 C9H8O3 164.161 —— meso-cis-5-norbornene-endo-2,3-diacetic acid dimethyl ester 92621-94-8 C13H18O4 238.284 —— (1R,2S,3R,4S)-bicyclo[2.2.1]hept-5-ene-2,3-dimethanol 941567-71-1 C9H14O2 154.209 —— endo-cis-Norbornan-2,3-dicarbonsaeure 2435-37-2 C9H12O4 184.192 —— cis,cis,cis,cis-1,2,3,4-cyclopentanetetracarboxylic acid 3786-91-2 C9H10O8 246.174 —— endo-2,3-diacetylbicyclo[2.2.1]hept-5-ene 3669-59-8 C11H14O2 178.231
反应信息
-
作为反应物:描述:5-降冰片烯-2,3-二羧酸 在 Tetrabutylammonium Rhodanide 作用下, 以 乙腈 为溶剂, 以60%的产率得到3,6-endomethylene-1,2,3,6-tetrahydrophthalic anhydride参考文献:名称:二元酸电化学脱水成环酸酐摘要:提出了二羧酸与其环状酸酐的电化学脱水反应。电化学产生的酸酐可直接用于酰胺化反应。通过18 O 同位素标记研究了反应机理,揭示了电解过程中硫酸盐的形成。DOI:10.1002/chem.202400403
-
作为产物:描述:3,6-endomethylene-1,2,3,6-tetrahydrophthalic anhydride 在 水 作用下, 以86%的产率得到5-降冰片烯-2,3-二羧酸参考文献:名称:使用烯烃和炔烃的复分解反应合成环状和大环醚摘要:已经研究了带有烯丙基和/或炔丙基醚取代基的环己烷和环己烯的复分解转化。使用第一代和第二代明确定义的亚烷基钌催化剂获得了一系列含有稠合 6,8-双环系统的产物,这些产物由烯烃和/或烯炔复分解产生。然而,无应变的环己烯单元不参与任何这些复分解过程,即使这样做会导致热力学更稳定的产品。这与之前对类似化合物和相应的氨基取代环己烯的结果形成对比。还报道了通过涉及合适环己烯醚侧链的复分解过程合成二烯和二氢呋喃。当无应变的环己烯单元被降冰片烯基团取代时,紧张的烯烃确实参与了烯烃和烯炔复分解的级联反应。这种化学反应扩展到环己烯和降冰片烯酯的炔烃复分解反应,产生 6,12-稠合的环炔烃,随后将进行进一步的烯炔和/或烯烃复分解反应。这最终导致了第一个单锅复分解过程的发展,在该过程中,由两种不同催化剂引发的炔烃、烯炔和烯烃复分解反应依次发生,形成 5,12-稠合双环二烯产物。(© Wiley-VCH VerlagDOI:10.1002/ejoc.200700291
文献信息
-
Epimerization of Tertiary Carbon Centers via Reversible Radical Cleavage of Unactivated C(sp<sup>3</sup>)–H Bonds作者:Yaxin Wang、Xiafei Hu、Cristian A. Morales-Rivera、Guo-Xing Li、Xin Huang、Gang He、Peng Liu、Gong ChenDOI:10.1021/jacs.8b05753日期:2018.8.1cleavage of C(sp3)-H bonds can enable racemization or epimerization, offering a valuable tool to edit the stereochemistry of organic compounds. While epimerization reactions operating via cleavage of acidic C(sp3)-H bonds, such as the Cα-H of carbonyl compounds, have been widely used in organic synthesis and enzyme-catalyzed biosynthesis, epimerization of tertiary carbons bearing a nonacidic C(sp3)-H bondC(sp3)-H 键的可逆断裂可以实现外消旋化或差向异构化,为编辑有机化合物的立体化学提供了宝贵的工具。虽然通过裂解酸性 C(sp3)-H 键(例如羰基化合物的 Cα-H)进行的差向异构化反应已广泛用于有机合成和酶催化生物合成,但带有非酸性 C(sp3) 的叔碳的差向异构化-H 键更具挑战性,可用的实用方法很少。在这里,我们报告了第一个合成有用的协议,用于在温和条件下通过未活化的 C(sp3)-H 键与高价碘试剂苯并恶唑叠氮化物和 H2O 的可逆自由基裂解来进行叔碳差向异构化。这些反应对各种环烷烃的未活化 3° CH 键表现出优异的反应性和选择性,并为编辑传统方法难以处理的碳支架的立体化学构型提供了强大的策略。机理研究表明,N3• 作为催化氢原子穿梭的独特能力对于以高效率和选择性可逆地破坏和重组 3° CH 键至关重要。
-
Structure-reactivity relations for thiol-disulfide interchange作者:Janette Houk、George M. WhitesidesDOI:10.1021/ja00256a040日期:1987.10di- and trithiols and the disulfides derived from either 2-mercaptoethanol or dithiothreitol. Reactions were conducted in methanol-d,/aqueous buffer (pH 7) or methanold, at 25 "C, using NMR spectroscopy to follow the reactions. These data were used to rank the dithiols in terms of reduction potential and to infer the structure of the disulfides formed from them on oxidation. There is a general correlation平衡常数由 36 个二硫醇和三硫醇与衍生自 2-巯基乙醇或二硫苏糖醇的二硫化物之间的硫醇-二硫化物交换确定。反应在甲醇-d/水性缓冲液 (pH 7) 或甲醇中进行,在 25°C 下,使用 NMR 光谱跟踪反应。这些数据用于根据还原电位对二硫醇进行排序并推断其结构氧化时由它们形成的二硫化物。二硫醇的还原能力与氧化时形成的含二硫化物环的大小之间存在普遍的相关性:形成六元环的二硫醇还原性最强(K = 103-105 M 相对于氧化的 2-巯基乙醇);五元和七元环的还原性降低约 1 个数量级。类似于 1 的化合物,2-乙二硫醇在相对稀释的溶液 (- 1 mM) 中形成环状双(二硫化物)二聚体,但在较高浓度下聚合。其他类别的二硫醇在氧化时形成聚合物。
-
Bislactonizations of olefinic diacids with [hydroxy(tosyloxy)iodo]benzene作者:Mayur Shah、Michael J. Taschner、Gerald F. Koser、Nancy L. Rach、Thomas E. Jenkins、Patrick Cyr、David PowersDOI:10.1016/s0040-4039(00)85231-4日期:1986.1The reactions of [hydroxy(tosyloxy)iodo]benzene with a series of olefinic diacids to produce bislactones are reported. The products are the result of a stereospecific -addition of the two carboxylic acid functions to the double bond.
-
Bislactonization of unsaturated diacids using lead tetraacetate作者:E.J. Corey、Andrew W. GrossDOI:10.1016/s0040-4039(00)92788-6日期:1980.1The reaction of lead tetraacetate with unsaturated carboxylic acids (or salts) to from bislactones (γ or δ) can be controlled to produce efficiently addition of two carboxylic oxygens to the double bond, in consonance with an initial plumbolactonization step followed by SN2 displacement of lead.
-
Iodine‐Catalyzed Diels‐Alder Reactions作者:Thiemo Arndt、Philip K. Wagner、Jonas J. Koenig、Martin BreugstDOI:10.1002/cctc.202100342日期:2021.6.18The Diels-Alder cycloaddition is the most popular pericyclic reaction with numerous applications in synthesis and catalysis. We now demonstrate that we can perform this reaction under mild and metal-free conditions relying on molecular iodine as the catalyst. Cycloadditions with cyclohexadiene, cyclopentadiene, or isoprene with various dienophiles can be performed typically within minutes in moderate
表征谱图
-
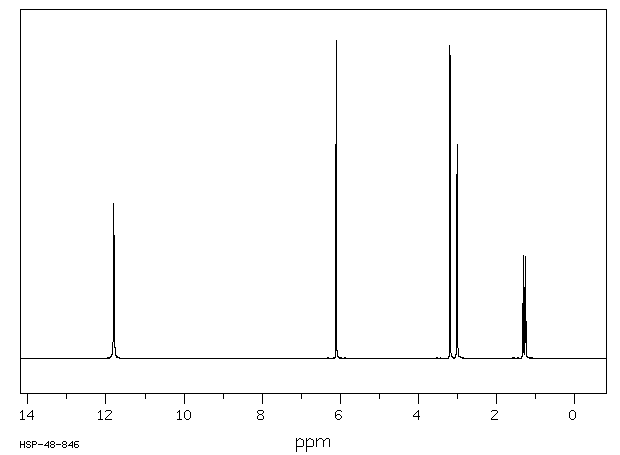
氢谱1HNMR
-
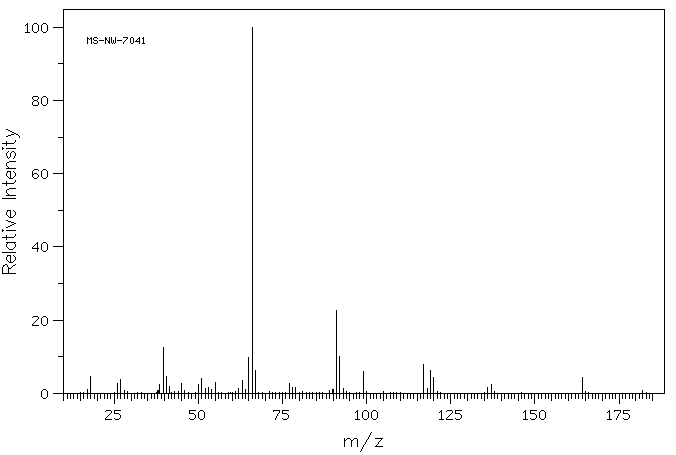
质谱MS
-
碳谱13CNMR
-
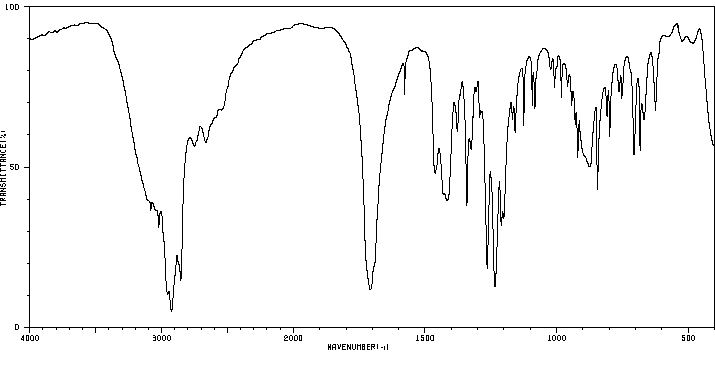
红外IR
-
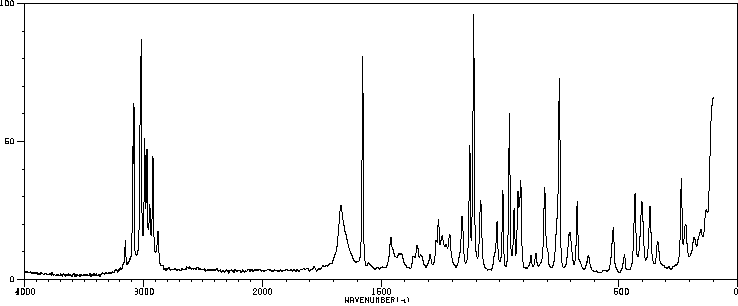
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸