7-(1-环庚-2,4,6-三烯基)环庚-1,3,5-三烯 | 831-18-5
中文名称
7-(1-环庚-2,4,6-三烯基)环庚-1,3,5-三烯
中文别名
——
英文名称
ditropenyl
英文别名
Bitropenyl;7-cyclohepta-2,4,6-trien-1-ylcyclohepta-1,3,5-triene
CAS
831-18-5
化学式
C14H14
mdl
——
分子量
182.265
InChiKey
DMVKBMNNLFFZBY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4.7
-
重原子数:14
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2902199090
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : 7,7'-BI(1,3,5-CYCLOHEPTATRIENE)
CAS-No. : 831-18-5
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008 [EU-GHS/CLP]
Acute toxicity, Oral (Category 4)
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Harmful if swallowed.
Label elements
Labelling according Regulation (EC) No 1272/2008 [CLP]
Pictogram
Signal word Warning
Hazard statement(s)
Harmful if swallowed.
Precautionary statement(s) none
Supplemental Hazard none
Statements
According to European Directive 67/548/EEC as amended.
Hazard symbol(s)
R-phrase(s)
R22 Harmful if swallowed.
S-phrase(s) none
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Formula : C14H14
Molecular Weight : 182,27 g/mol
Component Concentration
7,7'-BI(1,3,5-CYCLOHEPTATRIENE)
CAS-No. 831-18-5 -
Section 4. FIRST AID MEASURES
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Flush eyes with water as a precaution.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
To the best of our knowledge, the chemical, physical, and toxicological properties have not been thoroughly
investigated.
Indication of immediate medical attention and special treatment needed
no data available
Section 5. FIRE-FIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides
Precautions for fire-fighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid dust formation. Avoid breathing vapors, mist or gas. Ensure
adequate ventilation. Avoid breathing dust.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols.
Provide appropriate exhaust ventilation at places where dust is formed.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Specific end uses
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and at
the end of workday.
Personal protective equipment
Eye/face protection
Safety glasses with side-shields conforming to EN166 Use equipment for eye protection tested and
approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the
standard EN 374 derived from it.
Body Protection
Complete suit protecting against chemicals, The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
For nuisance exposures use type P95 (US) or type P1 (EU EN 143) particle respirator.For higher
level protection use type OV/AG/P99 (US) or type ABEK-P2 (EU EN 143) respirator cartridges. Use
respirators and components tested and approved under appropriate government standards such as
NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: solid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting/freezing point no data available
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Autoignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation May be harmful if inhaled. May cause respiratory tract irritation.
Ingestion Harmful if swallowed.
Skin
May be harmful if absorbed through skin. May cause skin irritation.
Eyes May cause eye irritation.
Signs and Symptoms of Exposure
To the best of our knowledge, the chemical, physical, and toxicological properties have not been thoroughly
investigated.
Additional Information
RTECS: Not available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN-Number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for users
no data available
Section 15. REGULATORY INFORMATION
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:7-(1-环庚-2,4,6-三烯基)环庚-1,3,5-三烯 在 氧气 作用下, 生成 tropenylperoxyl参考文献:名称:共缩合的金刚烷基质中的气相自由基:环庚三烯基过氧自由基的esr光谱中的质子分裂摘要:的环庚三烯基(tropenyl)基团是通过ditropenyl的气相热解在473-723ķ产生并在77K良好分辨的tropenyl的isotrppic ESR谱被困在共缩合金刚烷基基团呈的线性温度依赖性异在范围为77–226K。如果在存在双氧的情况下进行了二tropenyl的热解,则可得到tropenyl peroxyl自由基。在金刚烷基质中于77 K下获得的对苯二甲酰过氧自由基的各向异性ESR光谱的特征在于g 1 = 2.035,g 2 = 2.009,g 3 = 2.002和a 1 = 8.4 G,†a 2 = 9.6 G和a 3 = 5.35 G. g iso和a的值在130 K的自由旋转自由基中实验发现iso分别为g = 2.0157和a = 7.5G。超精细偶联起因于通过超共轭机理与β-H原子的偶联。DOI:10.1016/0040-4020(89)80115-2
-
作为产物:描述:参考文献:名称:环庚三烯与一些芳香醌的热加成反应:vic-ditropylation 产物的形成摘要:研究了环庚三烯与一些芳香醌的热加成反应。除了普通的 Diels-Alder 加合物外,在产品中还发现了 2,3-ditropylcyclohex-5-ene-1,4-diones,它们是在一系列的 tropylation、脱氢和进一步的 tropylation 过程后形成的。DOI:10.1246/cl.1976.445
文献信息
-
Sulfur Monoxide Transfer from<i>peri</i>-Substituted Trisulfide-2-oxides to Dienes: Substituent Effects, Mechanistic Studies and Application in Thiophene Synthesis作者:Richard S. Grainger、Bhaven Patel、Benson M. Kariuki、Louise Male、Neil SpencerDOI:10.1021/ja108865w日期:2011.4.20followed by in situ trapping by diene. Transfer of SO also occurs upon irradiation at room temperature, but yields of sulfoxide are lower. Dehydration of the sulfoxides under Pummerer conditions gives thiophenes, including the naturally occurring thioperillene. Two dienes form thiophenes directly under the SO transfer conditions. The methodology is applied in a formal synthesis of the antiplatelet medication通过用亚硫酰氯和吡啶处理 1,8-萘二硫醇制备了三个环取代的三硫化物-2-氧化物。1,2,3-trithiane-2-氧化物环在固态下采用沙发构象,具有假轴氧和环应变的证据(周边相互作用)。在二烯存在下加热三硫化物-2-氧化物会导致正式的一氧化硫 (SO) 转移形成不饱和环状亚砜,以及可回收的 1,8-萘二硫化物。萘环上邻甲氧基或邻叔丁基取代基的存在降低了温度并增加了 SO 转移发生的速率。捕获实验和动力学研究与三线态 SO 的产生一致,然后是二烯的原位捕获。SO 的转移也发生在室温下辐照时,但亚砜的收率较低。在Pummerer 条件下,亚砜脱水得到噻吩,包括天然存在的硫紫杉烯。两种二烯在 SO 转移条件下直接形成噻吩。该方法应用于抗血小板药物波立维的正式合成。
-
The Thermal Addition Reactions of Cycloheptatriene with Aromatic<i>p</i>-Quinones作者:Akira Mori、Hiroaki Mametsuka、Hitoshi TakeshitaDOI:10.1246/bcsj.58.2072日期:1985.7Thermal addition reactions of cycloheptatriene with several aromatic p-quinones gave the Diels-Alder adducts as minor products; the most characteristic feature was the formation of the vic-ditropylation products. The mechanism of their formation was clarified to be a sequential ene-reaction and dehydrogenation by means of chemical conversion from the isolated intermediates. Several new other additions
-
Synthese neuer Heptafulvene, Röntgenstrukturanalyse von ‘8,8-(1′,4′-Dioxotetramethylen)heptafulven’(2-(Cyclohepta-2,4,6-trien-1-yliden)cyclopentan-1,3-dion)作者:Peter Bönzli、Markus Neuenschwander、Peter EngelDOI:10.1002/hlca.19900730614日期:1990.9.19Synthesis of New Heptafulvenes; X-Ray Analysis of ‘8,8-(1′,4′-Dioxotetramethylene)heptafulvene’ (2-(Cyclohepta-2,4,6-trien-1-ylidene)cyclopentane-1,3-dione)
-
Facile decomposition of 9-substituted 9-xanthydrols in basic media. Dependence of reaction behavior on structure and metal ion作者:Rafik Karaman、Ibraheem T. Badejo、James L. FryDOI:10.1021/ja00198a082日期:1989.8have Rsub 3}COH yields} Rsub 2}C double bond} O + RH (1) decompositions of alcohols by free-radical pathways. Theoretical and experimental studies of the mechanisms of alkoxide decompositions have considered both heterolytic and homolytic pathways. Here they report the observation of unusual facile fragmentation of the alkoxides of several 9-substituted-9-xanthydrols in THF or Csub 6}Dsub 6} by空间拥挤的叔醇可能会碎裂成酮和烃(方程式 1)。已经报道了醇盐在凝聚相和气相中离子分解为碳负离子和羰基化合物,以及 Rsub 3}COH 产量} Rsub 2}C 双键} O + RH (1) 分解醇通过自由基途径。醇盐分解机制的理论和实验研究已经考虑了异裂和均裂途径。在这里,他们报告了在 THF 或 Csub 6}Dsub 6} 中通过离子或自由基过程观察到的几种 9-取代-9-xanthydrols 的醇盐的异常容易碎裂,这取决于取代基的性质和金属。
-
The One-electron Reduction of Carbonium Ions. VI. The Trapping of the Cycloheptatrienyl Radical with 2-Methyl-2-nitrosopropane in the Course of the Zinc Reduction of the Tropylium Ion作者:Kunio Okamoto、Koichi KomatsuDOI:10.1246/bcsj.47.1709日期:1974.7pane (a radical scavenger) in H2O–THF (7:3 by volume) at room temperature. In the reaction mixture with a short reaction time (5 min), t-butyl tropyl nitroxide (II) (a radical-trapping product) was identified by the ESR analysis. α-Phenyl-N-t-butylnitrone (I), which was isolated from the reaction mixture during a prolonged reaction time (30 min), was proved to be derived from the nitroxide II through
表征谱图
-
氢谱1HNMR
-
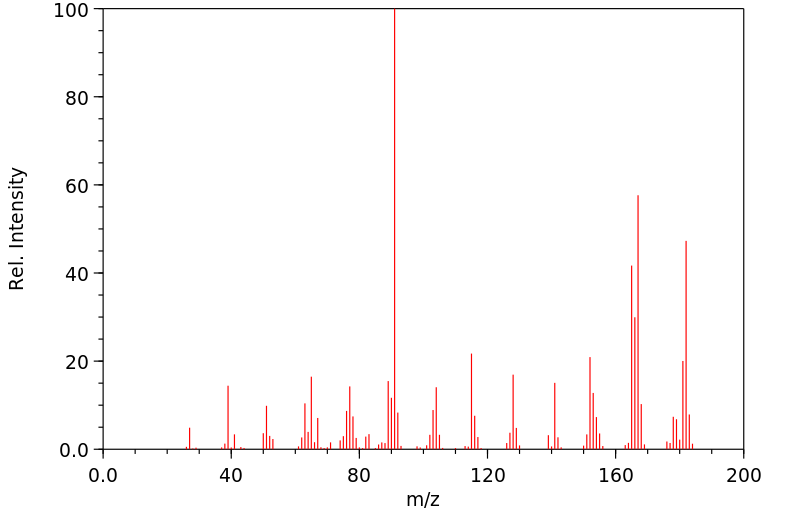
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-