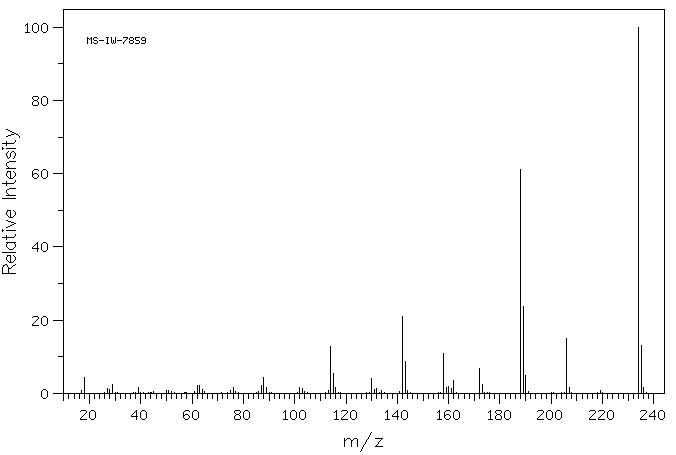
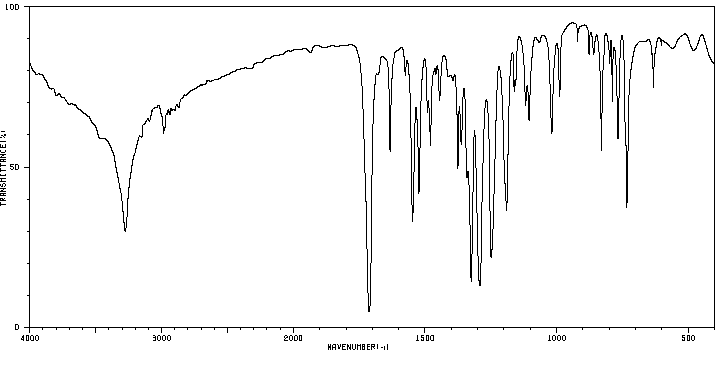
7-硝基吲哚-2-甲酸乙酯 | 6960-46-9
中文名称
7-硝基吲哚-2-甲酸乙酯
中文别名
7-硝基吲哚-2-羧酸乙酯
英文名称
ethyl 7-nitroindole-2-carboxylate
英文别名
ethyl 7-nitro-1H-indole-2-carboxylate
CAS
6960-46-9
化学式
C11H10N2O4
mdl
MFCD00216475
分子量
234.211
InChiKey
GTZAIVBXGPLYGD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:90-96 °C
-
沸点:376.52°C (rough estimate)
-
密度:1.3240 (rough estimate)
-
稳定性/保质期:
在常温常压下保持稳定,应避免与不相容的材料以及光照接触。
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:17
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.181
-
拓扑面积:87.9
-
氢给体数:1
-
氢受体数:4
安全信息
-
危险品标志:Xi
-
海关编码:2933990090
-
安全说明:S24/25
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
SDS
| Name: | Ethyl 7-nitroindole-2-carboxylate 95+% Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 6960-46-9 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 6960-46-9 | Ethyl 7-nitroindole-2-carboxylate | 95+ | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 6960-46-9: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: pale yellow
Odor: none reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 90.00 - 92.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water: negligible
Specific Gravity/Density: Not available.
Molecular Formula: C11H10N2O4
Molecular Weight: 234.21
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Irritating and toxic fumes and gases.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 6960-46-9 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Ethyl 7-nitroindole-2-carboxylate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 6960-46-9: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 6960-46-9 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 6960-46-9 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 7-硝基吲哚-2-甲酸 7-nitro-1H-indole-2-carboxylic acid 6960-45-8 C9H6N2O4 206.158 —— 7-nitroindole-2-carbaldehyde —— C9H6N2O3 190.158 乙基7-氨基-1H-吲哚-2-羧酸酯 ethyl 7-amino-1H-indole-2-carboxylate 71056-61-6 C11H12N2O2 204.228 —— ethyl 3-bromo-7-nitro-1H-indole-2-carboxylate 411230-09-6 C11H9BrN2O4 313.107 —— Ethyl 1-methyl-7-nitro-2-indolecarboxylate 167478-87-7 C12H12N2O4 248.238 —— 7-nitro-1H-indole-2-carboxamide 90886-63-8 C9H7N3O3 205.173 —— 1-methyl-7-nitroindole-2-carboxylic acid 616238-88-1 C10H8N2O4 220.185 —— 3-ethyl-7-nitro-1H-indole-2-carboxylic acid ethyl ester 71319-83-0 C13H14N2O4 262.265 —— 7-nitro-1H-indole-2-carbohydrazide 90886-84-3 C9H8N4O3 220.188 —— ethyl 7-acetamido-1H-indole-2-carboxylate 154422-24-9 C13H14N2O3 246.266 —— ethyl 1-(methoxymethyl)-7-nitro-1H-indole-2-carboxylate 913286-04-1 C13H14N2O5 278.265 —— 3-isobutyl-7-nitro-1H-indole-2-carboxylic acid 1579994-35-6 C13H14N2O4 262.265 —— 7-nitro-3-phenyl-1H-indole-2-carboxylic acid 121983-01-5 C15H10N2O4 282.255 —— 3-(3-fluorophenyl)-7-nitro-1H-indole-2-carboxylic acid 1579994-38-9 C15H9FN2O4 300.246 —— 3-(3-methoxyphenyl)-7-nitro-1H-indole-2-carboxylic acid 1579994-36-7 C16H12N2O5 312.282 —— 3-(3-acetamidophenyl)-7-nitro-1H-indole-2-carboxylic acid 1579994-37-8 C17H13N3O5 339.307 7-硝基吲哚 7-nitro-1H-indole 6960-42-5 C8H6N2O2 162.148 7-氯吲哚-2-甲酸乙酯 ethyl 7-chloro-1H-indole-2-carboxylate 43142-64-9 C11H10ClNO2 223.659 —— 2-[(3,5-dimethyl-1H-pyrazol-1-yl)carbonyl]-7-nitro-1H-indole 1221418-46-7 C14H12N4O3 284.274 —— ethyl 6,8-bis(trifluoromethyl)-1H-pyrrolo[3,2-h]quinoline-2-carboxylate 1431426-38-8 C16H10F6N2O2 376.258 - 1
- 2
反应信息
-
作为反应物:描述:7-硝基吲哚-2-甲酸乙酯 在 一水合肼 、 potassium hydroxide 作用下, 以 甲醇 为溶剂, 反应 5.0h, 生成 5-(7-nitro-1H-indol-2-yl)-1,3,4-oxadiazole-2-thiol参考文献:名称:Narayana; Ashalatha; Vijaya Raj, Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 2009, vol. 48, # 12, p. 1794 - 1805摘要:DOI:
-
作为产物:描述:(E)-ethyl pyruvate 2-(2-nitrophenyl)hydrazone 在 PPA 作用下, 反应 0.5h, 以78%的产率得到7-硝基吲哚-2-甲酸乙酯参考文献:名称:Fischer Indolization of Variously ortho-Substituted Phenylhydrazones. Fischer Indolization and Its Related Compounds. XXV.摘要:各种丙酮酸乙酯 2-(2-取代苯基)酰肼(1)在酸催化剂作用下进行费歇尔吲哚化反应。所有的苯肼都得到了相应的 7-取代吲哚(2)。此外,如果苯肼的正位取代基是电子疏导型的,或者其中心原子上有一个未共享的电子对,则往往会产生 4 或 5 取代的吲哚(3),这是由正位取代基在环化过程中发生迁移而产生的;而如果苯肼的正位取代基是电子吸引型的,则往往很少或根本不会产生这种异常产物。所使用的酸催化剂对产物的产率有一定影响,但对产物的种类没有影响。DOI:10.1248/cpb.41.1910
文献信息
-
Indole compound申请人:Yasuma Tsuneo公开号:US20080096877A1公开(公告)日:2008-04-24The purpose of the present invention is to provide a glucokinase activator useful as a pharmaceutical agent such as an agent for the prophylaxis or treatment of diabetes, obesity and the like. The present invention provides a glucokinase activator containing a compound represented by the formula (I): wherein R 1 is a hydrogen atom or a halogen atom; R 2 is a group represented by wherein each symbol is defined in the specification, or a salt thereof or a prodrug thereof.
-
N-酰基磺酰胺类FBPase抑制剂、其制备方法、 药物组合物及用途
-
Structure-based discovery of 1H-indole-2-carboxamide derivatives as potent ASK1 inhibitors for potential treatment of ulcerative colitis作者:Shaohua Hou、Xiping Yang、Yu Tong、Yuejing Yang、Quanwei Chen、Boheng Wan、Ran Wei、Yuchen Wang、Yanmin Zhang、Bo Kong、Jianhang Huang、Yadong Chen、Tao Lu、Qinghua Hu、Ding DuDOI:10.1016/j.ejmech.2020.113114日期:2021.2compound 19 with a novel indole-2-carboxamide hinge scaffold. Compound 19 displays potent anti-ASK1 kinase activity and stronger inhibitory effect on ASK1 in AP1-HEK293 cells than previously described ASK1 inhibitor GS-4997. Besides improved in vitro activity, compound 19 also exhibits an appropriate in vivo PK profile. In a dextran sulfate sodium (DSS)-induced mouse model of ulcerative colitis (UC), compound凋亡信号调节激酶1(ASK1)是丝裂原激活的蛋白激酶(MAPK)家族的成员,与许多人类疾病有关。在这里,我们描述了命中化合物7的结构优化,并进行了进一步的结构-活性关系(SAR)研究,从而开发了带有新型吲哚-2-羧酰胺铰链支架的化合物19。与先前描述的ASK1抑制剂GS-4997相比,化合物19在AP1-HEK293细胞中显示出强大的抗ASK1激酶活性和对ASK1的更强抑制作用。除了改善体外活性外,化合物19还具有适当的体内活性PK配置文件。在右旋糖酐硫酸钠(DSS)诱导的溃疡性结肠炎(UC)小鼠模型中,化合物19显示出显着的抗UC功效,并显着减轻了DSS诱导的体重减轻,结肠缩短,疾病活动指数(DAI)升高和炎症结肠组织中的细胞浸润。从机理上讲,化合物19抑制ASK1-p38 / JNK信号通路的磷酸化,并抑制炎症细胞因子的过表达。这些发现共同表明,ASK1抑制剂可以潜在地用作UC的治疗策略。
-
Indoles via Knoevenagel–Hemetsberger reaction sequence作者:William L. Heaner IV、Carol S. Gelbaum、Leslie Gelbaum、Pamela Pollet、Kent W. Richman、William DuBay、Jeffrey D. Butler、Gregory Wells、Charles L. LiottaDOI:10.1039/c3ra42296h日期:——sequential reaction of aromatic aldehydes with ethyl azidoacetate in the presence of sodium ethoxide to form the corresponding ethyl α-azido-β-arylacrylates (Knoevenagel process) followed by a solvent mediated thermolysis (Hemetsberger process). The isolated yields of the ethyl α-azido-β-arylacrylates were significantly increased when employing the sacrificial electrophile ethyl trifluoroacetate. 1H NMR and通过芳族醛与环戊烯的顺序反应合成了一系列取代的吲哚 叠氮基乙酸乙酯 在......的存在下 乙醇钠形成相应的乙基α-叠氮基-β-芳基丙烯酸乙酯(Knoevenagel法),然后进行溶剂介导的热分解(Hemetsberger法)。使用牺牲亲电试剂时,α-叠氮基-β-芳基丙烯酸乙酯的分离收率显着提高三氟乙酸乙酯。对α-叠氮基-β-芳基丙烯酸乙酯的1 H NMR和1 H- 13 C NMR耦合分析表明,该缩合反应具有立体特异性,只能检测到Z异构体。溶剂介导的间位取代的乙基α-叠氮基-β-芳基丙烯酸酯的热处理导致5位和7位取代的吲哚的形成,其中5位区域异构体比7位区域异构体略受青睐。(2 Z,2 Z ')-二乙基3,3'-(1,3-亚苯基)双(2-叠氮基丙烯酸酯)和(2 Z,2 Z ')-二乙基3,3'-(1的相似热处理,4-亚苯基)双(2-叠氮基丙烯酸酯)专门生产吡咯并吲哚,1,5-二氢吡咯并[2
-
INDOLE COMPOUNDS AS AN INHIBITOR OF CELLULAR NECROSIS申请人:Kim Soon Ha公开号:US20100210647A1公开(公告)日:2010-08-19The present invention relates to new indole compounds, pharmaceutically acceptable salts or isomers thereof which are useful for the prevention or treatment of cellular necrosis and necrosis-associated diseases. The present invention also relates to a method and a composition for the prevention or treatment of cellular necrosis and necrosis-associated diseases, comprising said indole compounds as an active ingredient.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Z)-3-[[[2,4-二甲基-3-(乙氧羰基)吡咯-5-基]亚甲基]吲哚-2--2-
(S)-(-)-5'-苄氧基苯基卡维地洛
(R)-(+)-5'-苄氧基卡维地洛
(R)-卡洛芬
(N-(Boc)-2-吲哚基)二甲基硅烷醇钠
(E)-2-氰基-3-(5-(2-辛基-7-(4-(对甲苯基)-1,2,3,3a,4,8b-六氢环戊[b]吲哚-7-基)-2H-苯并[d][1,2,3]三唑-4-基)噻吩-2-基)丙烯酸
(4aS,9bR)-6-溴-2,3,4,4a,5,9b-六氢-1H-吡啶并[4,3-B]吲哚
(3Z)-3-(1H-咪唑-5-基亚甲基)-5-甲氧基-1H-吲哚-2-酮
(3Z)-3-[[[4-(二甲基氨基)苯基]亚甲基]-1H-吲哚-2-酮
(3R)-(-)-3-(1-甲基吲哚-3-基)丁酸甲酯
(3-氯-4,5-二氢-1,2-恶唑-5-基)(1,3-二氧代-1,3-二氢-2H-异吲哚-2-基)乙酸
齐多美辛
鸭脚树叶碱
鸭脚木碱,鸡骨常山碱
鲜麦得新糖
高氯酸1,1’-二(十六烷基)-3,3,3’,3’-四甲基吲哚碳菁
马鲁司特
马鞭草(VERBENAOFFICINALIS)提取物
马来酸阿洛司琼
马来酸替加色罗
顺式-ent-他达拉非
顺式-1,3,4,4a,5,9b-六氢-2H-吡啶并[4,3-b]吲哚-2-甲酸乙酯
顺式-(+-)-3,4-二氢-8-氯-4'-甲基-4-(甲基氨基)-螺(苯并(cd)吲哚-5(1H),2'(5'H)-呋喃)-5'-酮
靛青二磺酸二钾盐
靛藍四磺酸
靛红联二甲酚
靛红磺酸钠
靛红磺酸
靛红乙烯硫代缩酮
靛红-7-甲酸甲酯
靛红-5-磺酸钠
靛红-5-磺酸
靛红-5-硫酸钠盐二水
靛红-5-甲酸甲酯
靛红
靛玉红衍生物E804
靛玉红3'-单肟5-磺酸
靛玉红-3'-单肟
靛玉红
靛噻
青色素3联己酸染料,钾盐
雷马曲班
雷莫司琼杂质13
雷莫司琼杂质12
雷莫司琼杂质
雷替尼卜定
雄甾-1,4-二烯-3,17-二酮
阿霉素的代谢产物盐酸盐
阿贝卡尔
阿西美辛杂质3