Alpha-(二甲基氯化硅烷)异丙苯 | 118740-38-8
中文名称
Alpha-(二甲基氯化硅烷)异丙苯
中文别名
Α-(氯代二甲硅基)枯烯
英文名称
chloro(α,α-dimethylbenzyl)dimethylsilane
英文别名
(1-methyl-1-phenylethyl)(dimethyl)chlorosilane;cumyldimethylsilyl chloride;chlorocumyldimethylsilane;dimethyl(phenyl dimethyl carbinyl)chlorosilane;Chlorodimethyl(2-phenylpropan-2-yl)silane;chloro-dimethyl-(2-phenylpropan-2-yl)silane
CAS
118740-38-8
化学式
C11H17ClSi
mdl
MFCD00143579
分子量
212.794
InChiKey
SUMFNCSNBLYOEK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
密度:1,01 g/cm3
计算性质
-
辛醇/水分配系数(LogP):4.18
-
重原子数:13
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.454
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
反应信息
-
作为反应物:描述:Alpha-(二甲基氯化硅烷)异丙苯 在 N-甲基吗啉 、 硝酸铵 、 氢氧化钾 、 草酰氯 、 溶剂黄146 、 N,N-二甲基甲酰胺 、 三氟乙酸酐 、 sodium iodide 、 锌 作用下, 以 四氢呋喃 、 甲醇 、 二氯甲烷 、 N,N-二甲基甲酰胺 、 乙腈 为溶剂, 反应 21.33h, 生成 N-(9-fluorenylmethoxycarbonyl)-O-[(4-amino-α,α-dimethylbenzyl)dimethylsilyl]-L-serine allyl ester参考文献:名称:A novel silyl linker: Motif for side chain tethered approach to solid-phase glycopeptide synthesis摘要:In order to facilitate the solid-phase syntheses of protected glycopeptide blocks, we designed a novel silyl linker, which allows the alcoholic side chain (carbohydrate, serine, or threonine) of (glyco-)peptides to link to the solid support. Utilizing this linker, peptide coupling reactions at both the N- and the C-termini were successful. Synthesis of the glycophorin AM fragment corresponding to the N-terminal glycoheptapeptide is demonstrated. (C) 1999 Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0040-4020(99)00649-3
-
作为产物:描述:参考文献:名称:ISIXARA, TOSINOBU;TAKAMIDZAVA, MINORU;EHNDO, MIKIO;KUBOTA, TORU摘要:DOI:
文献信息
-
Zinc Mediated Reactions in Organic Synthesis: Efficient Synthesis of Silyl Ethers under Mild Conditions
-
Total synthesis of buergerinin F via effective construction of the asymmetric quaternary carbons using an enantioselective aldol reaction作者:Isamu Shiina、Yo-ichi Kawakita、Ryoutarou Ibuka、Kazutoshi Yokoyama、Yu-suke YamaiDOI:10.1039/b507401k日期:——An efficient method for the synthesis of (+)-buergerinin F is established via the enantioselective aldol reaction of a tetra-substituted ketene silyl acetal with crotonaldehyde, followed by intramolecular Wacker-type ketalization.
-
Construction of AB-Ring System of Taxane Framework by A-Ring Annulation Strategy: Synthesis of 1-Hydroxy-8,11,11-trimethylbicyclo-(5.3.1)undec-7-en-9-one by Way of Intramolecular Aldol Cyclization to Form the C1-C10 Bond.作者:Koji YAMADA、Hayato IWADARE、Teruaki MUKAIYAMADOI:10.1248/cpb.45.1898日期:——Construction of the AB-ring system of the taxane framework via an A-ring annulation strategy was demonstrated by base-mediated intramolecular aldol reaction of (Z)-2,2-dimethyl-3-(1-methyl-2-oxopropylidene)cyclooctanone, affording the title compound, 1-hydroxy-8,11,11-trimethylbicyclo[5.3.1]undec-7-en-9-one. A cyclization precursor, the tetra-substituted (Z)-alkene, was prepared from the corresponding
-
Synthesis of AB Ring Model System of Taxol<i>via</i>Allylation of 8-Membered Ring Compound and Intramolecular Aldol Condensation作者:Teruaki Mukaiyama、Isamu Shiina、Kaname Kimura、Yuji Akiyama、Hayato IwadareDOI:10.1246/cl.1995.229日期:1995.31-t-Butyldimethylsiloxy-8,11,11-trimethylbicyclo[5.3.1]undec-7-en-9-one (2) has been synthesized via intramolecular aldol condensation of the precursor 3 by combined use of lithium diisopropylamide (LDA) and CeCl3. The dicarbonyl compound 3 was prepared by allylation of 8-membered ring ketone 6b.
-
Stereoselective Synthesis of Highly Substituted γ-Lactams by the [3+2] Annulation of α-Siloxy Allylic Silanes with Chlorosulfonyl Isocyanate作者:Antonio Romero、K. A. WoerpelDOI:10.1021/ol060596g日期:2006.5.1A stereoselective synthesis of gamma-lactams by the [3+2] annulation of alpha-siloxy allylic silanes with N-chlorosulfonyl isocyanate (CISO2NCO) was developed. The use of these allylic silanes allowed for further diastereoselective substitution of the resultant N,O-acetal to give highly substituted gamma-lactams. Oxidation of the silyl group afforded access to complex beta-hydroxy-gamma-lactams.
表征谱图
-
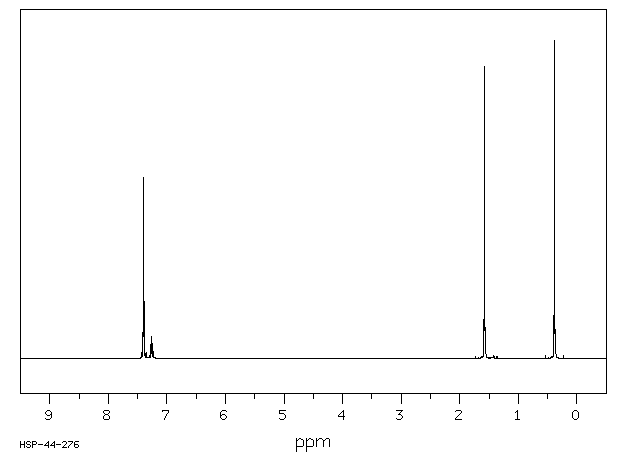
氢谱1HNMR
-
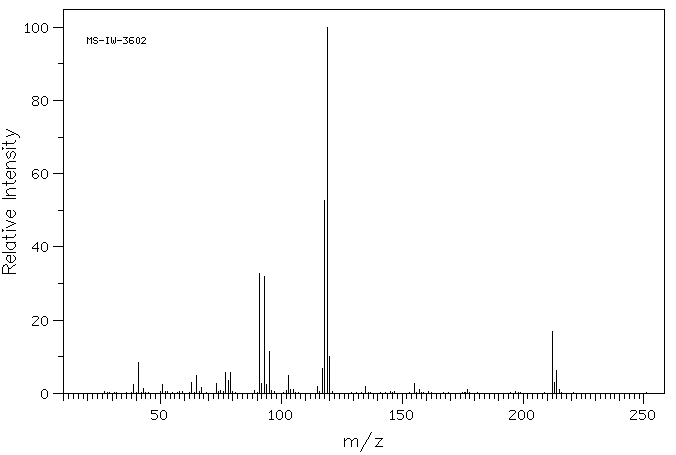
质谱MS
-
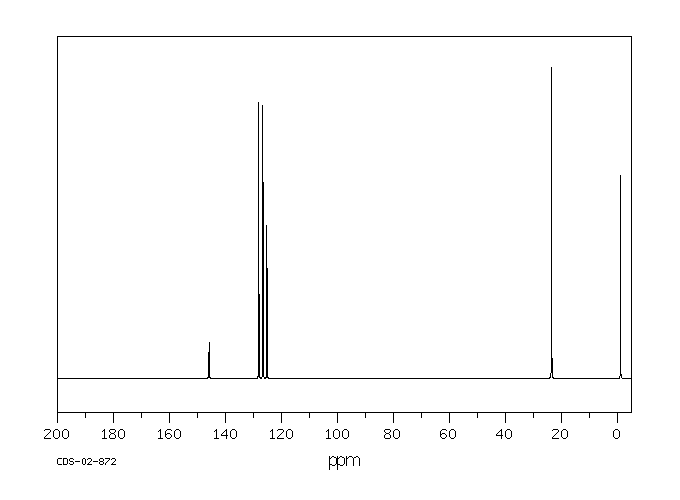
碳谱13CNMR
-
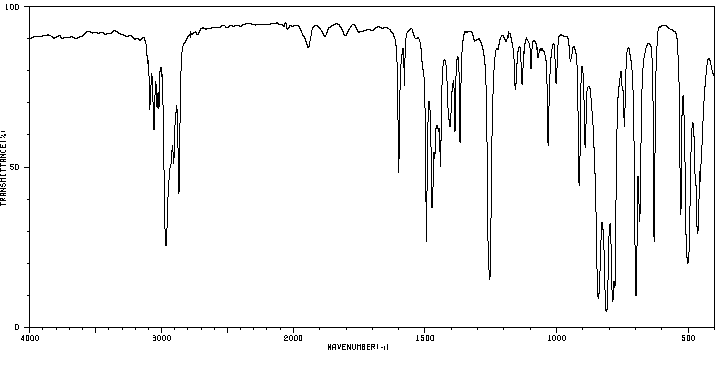
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫