Boc-D-天冬氨酸 | 62396-48-9
中文名称
Boc-D-天冬氨酸
中文别名
N-叔丁氧羰基-D-天冬氨酸;BOC-D-天门冬氨酸;N-(叔丁氧羰基)-D-天冬氨酸;Boc-D-Asp-OH
英文名称
Boc-D-Asp-OH
英文别名
Boc-D-Asp;N-Boc-D-Asp;Boc-D-Aspartic acid;(2R)-2-[(2-methylpropan-2-yl)oxycarbonylamino]butanedioic acid
CAS
62396-48-9
化学式
C9H15NO6
mdl
——
分子量
233.221
InChiKey
KAJBMCZQVSQJDE-RXMQYKEDSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:377.4±32.0 °C(Predicted)
-
密度:1.302±0.06 g/cm3(Predicted)
-
稳定性/保质期:
远离氧化物、光和热。
计算性质
-
辛醇/水分配系数(LogP):0.1
-
重原子数:16
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:0.67
-
拓扑面积:113
-
氢给体数:3
-
氢受体数:6
安全信息
-
安全说明:S24/25
-
海关编码:29225090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:存放在密封容器内,并放置在阴凉、干燥处。储存地点应远离氧化剂,避免阳光直射和高温。
SDS
N-(叔丁氧羰基)-D-天冬氨酸 修改号码:5
模块 1. 化学品
产品名称: N-(tert-Butoxycarbonyl)-D-aspartic Acid
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): N-(叔丁氧羰基)-D-天冬氨酸
百分比: >98.0%(LC)(T)
CAS编码: 62396-48-9
俗名: N-Boc-D-aspartic Acid , Boc-D-Asp-OH
分子式: C9H15NO6
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
N-(叔丁氧羰基)-D-天冬氨酸 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
N-(叔丁氧羰基)-D-天冬氨酸 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
N-(叔丁氧羰基)-D-天冬氨酸 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: N-(tert-Butoxycarbonyl)-D-aspartic Acid
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): N-(叔丁氧羰基)-D-天冬氨酸
百分比: >98.0%(LC)(T)
CAS编码: 62396-48-9
俗名: N-Boc-D-aspartic Acid , Boc-D-Asp-OH
分子式: C9H15NO6
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
N-(叔丁氧羰基)-D-天冬氨酸 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
N-(叔丁氧羰基)-D-天冬氨酸 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
N-(叔丁氧羰基)-D-天冬氨酸 修改号码:5
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 Boc-DL-天冬氨酸 N-Boc-aspartic acid 120341-32-4 C9H15NO6 233.221
反应信息
-
作为反应物:描述:参考文献:名称:Rediscovering an Endothelin Antagonist (BQ-123): A Self-Deconvoluting Cyclic Pentapeptide Library摘要:A ''self-deconvoluting'' cyclic pentapeptide library, designed to produce 82 944 head-to-tail-linked peptides in 48 vials, has been prepared. The mixture included amino acids found in a recently optimized endothelin antagonist, BQ-123, originally isolated from microbial sources bg Banyu investigators. Using a positional scan approach, the most potent of 12 residues at each of the four variable positions uniquely rediscovered the BQ-123 sequence or cyclo(L-Pro-D-Val-L-Leu-D-Trp-D-Asp). Resynthesis of the four most potent amino acid combinations gave the following values of relative potency: cyclo(L-Pro-D-Val-L-Leu-D-Trp-D-Asp) or BQ-123 = 1.0, cyclo(L-Pro-D-Pro-L-Leu-D-Trp-D-Asp) = 0.0, cyclo(L-Pro-D-Pro-L-Trp-D-Trp-D-Asp) = 0.0, and cyclo(L-Pro-D-Val-L-Trp-D-Trp-D-Asp) = 0.1. This study reflects the first time that the positional scan approach has been applied to cyclic peptide libraries using a known target, Although no analogs more potent than BQ-123 were discovered, our results provide verification of our synthetic methods for preparing head-to-tail cyclic peptide libraries and also lend support to the use of carefully designed sublibraries for the rapid elucidation of potential leads within a relatively constrained set of peptide macrocycles.DOI:10.1021/jm9604078
-
作为产物:描述:参考文献:名称:CANTACUZENE, D.;PASCAL, F.;GUERREIRO, C., TETRAHEDRON, 43,(1987) N 8, 1823-1826摘要:DOI:
文献信息
-
Synthesis and stability evaluation of novel peptidomimetic Caspase-1 inhibitors for topical application作者:Sandrine Chambon、Sandrine Talano、Corinne Millois、Laurence Dumais、Romain Pierre、Loic Tomas、Céline Mathieu、Anne-Laurence Ghilini、Nicolas Vanthuyne、Kevin Reverse、Anne Brethon、Vincent Rodeschini、Catherine Comino、Grégoire Mouis、Ghizlane El-Bazbouz、Laurence Clary、Jean-François Fournier、Claire Bouix-Peter、Craig S. Harris、Laurent F. HennequinDOI:10.1016/j.tet.2018.07.029日期:2018.9During our search for topically-active Caspase-1 inhibitors, we identified a novel class of potent inhibitors based on a 1,3,5-trisubstituted uracil motif equipped with an l-aspartate semi-aldehyde derived warhead. In the literature, the majority of Caspase-1 inhibitors possessing the same warhead have been designed and evaluated for oral administration as the ethyl acetal pro-drug form. For our topical
-
NOVEL MORPHOLINE DERIVATIVE OR SALT THEREOF申请人:FUJIFILM Corporation公开号:US20160168139A1公开(公告)日:2016-06-16There is provided a morpholine derivative represented by General Formula [1A] or a salt thereof. (In the formula, a ring A represents a ring represented by General Formula [I]; * represents a bonding position; Z 2 represents CH or the like; Z 1 represents CR 6 or the like; R 6 represents a hydrogen atom or the like; X 1 represents CHR 7 or the like; R 7 represents a hydrogen atom or the like; X 2 represents CH 2 or the like; R 1 and R 2 are the same as or different from each other, and each of R 1 and R 2 represents a hydrogen atom or the like; R 3 , R 4 , and R 5 are the same as or different from each other, and each of R 3 , R 4 , and R 5 represents a hydrogen atom, NR a R b , or the like; and each of R a and R b represents a hydrogen atom, a C 1-8 alkyl group which may have a substituent, or the like.)提供一种由通用式[1A]表示的吗啉衍生物或其盐。 (在该式中,环A代表由通用式[I]表示的环;*代表连接位置;Z 2 代表CH或类似物;Z 1 代表CR 6 或类似物;R 6 代表氢原子或类似物;X 1 代表CHR 7 或类似物;R 7 代表氢原子或类似物;X 2 代表CH 2 或类似物;R 1 和R 2 相同或不同,且R 1 和R 2 中的每一个代表氢原子或类似物;R 3 ,R 4 和R 5 相同或不同,且R 3 ,R 4 和R 5 中的每一个代表氢原子,NR a R b 或类似物;R a 和R b 中的每一个代表氢原子,可能具有取代基的C 1-8 烷基基团,或类似物。)
-
Inhibitors of protein tyrosine phosphatase申请人:Pharmacia & Upjohn Company公开号:US06353023B1公开(公告)日:2002-03-05The present invention comprises small molecular weight, non-peptidic inhibitors of formula I and II of Protein Tyrosine Phosphatase 1 (PTP1) which are useful for the treatment and/or prevention of Non-Insulin Dependent Diabetes Mellitus (NIDDM).
-
Morphological control of self-assembled multivalent (SAMul) heparin binding in highly competitive media
-
3-(Imidazolyl)-2-aminopropanoic acids申请人:Pfizer Inc.公开号:US20030191107A1公开(公告)日:2003-10-09Compounds according to formula (I) wherein n is 1-4, R 1 is optionally substituted C 1-6 alkyl, C 2-6 alkenyl, or C 2-6 alkynyl, Heterocycle, Aromatic heterocycle, Aryl or hydrogen and R 2 , R 3 , R 4 , R 5 , R 6 , R 7 and R 8 are each independently selected from hydrogen and optionally substituted C 1-6 alkyl are novel. They are useful in the treatment of thrombotic conditions and other pathologies associated with fibrin deposition. 1根据公式(I),其中n为1-4,R1是可选取代的C1-6烷基、C2-6烯基或C2-6炔基、杂环、芳香杂环、芳基或氢,而R2、R3、R4、R5、R6、R7和R8各自独立选择自氢和可选取代的C1-6烷基的化合物是新颖的。它们在治疗血栓症和与纤维蛋白沉积相关的其他病理情况中非常有用。
表征谱图
-
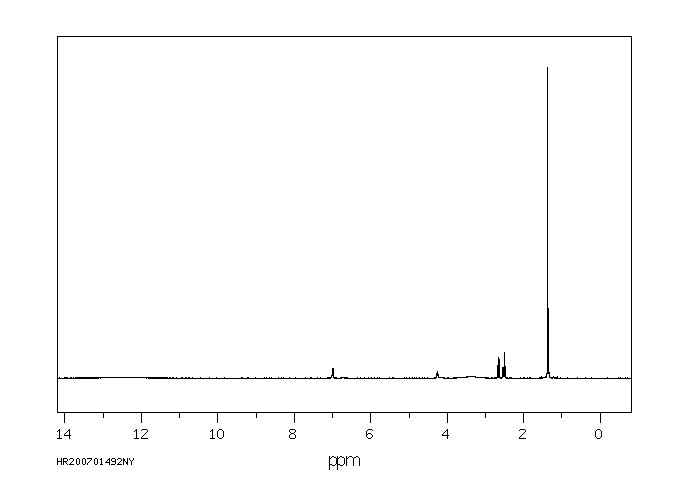
氢谱1HNMR
-
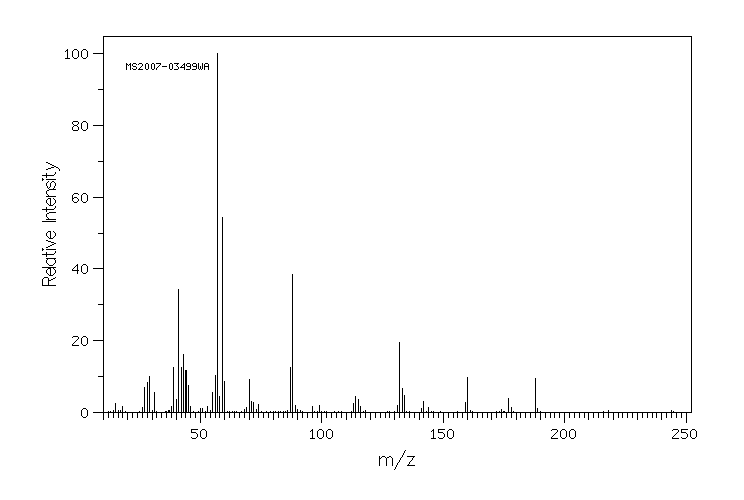
质谱MS
-
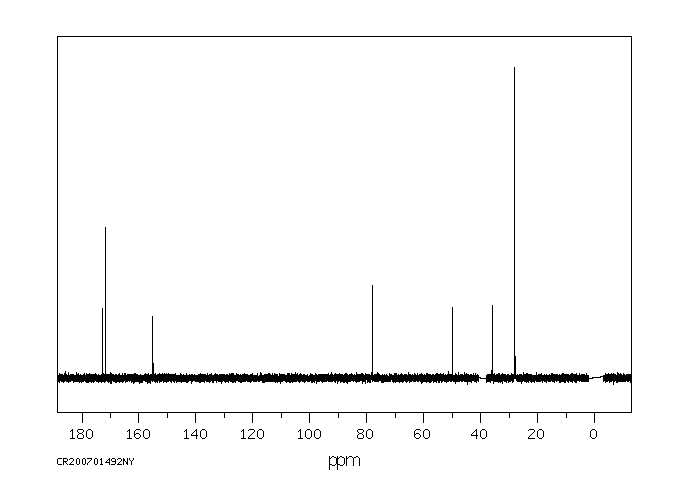
碳谱13CNMR
-
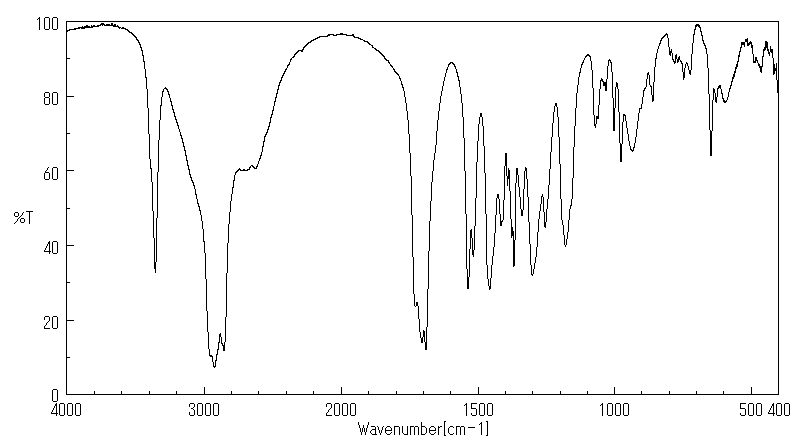
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸