L-苯丙氨酸乙酯盐酸盐 | 3182-93-2
中文名称
L-苯丙氨酸乙酯盐酸盐
中文别名
L-苯基丙氨酸乙酯盐酸盐;H-Phe-OEt・HCl
英文名称
(L)-phenylalanine ethyl ester hydrochloride
英文别名
phenylalanine ethyl ester hydrochloride;(S)-phenylalanine ethyl ester hydrochloride;ethyl L-phenylalaninate hydrochloride;ethyl (L)-phenylalanine hydrochloride;L-Phe-OEt hydrochloride;(S)-ethyl 2-amino-3-phenylpropanoate hydrochloride;ethyl (S)-phenylalaninate hydrochloride;H-Phe-OEt hydrochloride;H-Phe-OEt*HCl;Phe-OEt*HCl;H-(L)-Phe-OEt*HCl;L-Phe-OEt*HCl;ethyl (2S)-2-amino-3-phenylpropanoate;hydron;chloride
CAS
3182-93-2
化学式
C11H16NO2*Cl
mdl
MFCD00012507
分子量
229.707
InChiKey
FPFQPLFYTKMCHN-PPHPATTJSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:155-156 °C(lit.)
-
比旋光度:33.7 º (c=2, C2H5OH 21 ºC)
-
溶解度:溶于甲醇、水
计算性质
-
辛醇/水分配系数(LogP):1.58
-
重原子数:15
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.363
-
拓扑面积:52.3
-
氢给体数:2
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
安全说明:S22,S24/25,S26
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:29224995
-
危险标志:GHS07
-
危险性描述:H315,H319,H335
-
危险性防范说明:P261,P305 + P351 + P338
SDS
反应信息
-
作为反应物:描述:L-苯丙氨酸乙酯盐酸盐 在 sodium tetrahydroborate 、 sodium carbonate 、 lithium chloride 作用下, 以 1,4-二氧六环 、 乙醇 、 水 为溶剂, 反应 21.0h, 生成 BOC-L-苯丙氨酸参考文献:名称:轻松获取 Evans 的恶唑烷酮。一种新型2-恶唑烷酮衍生物的立体选择性合成及抗菌活性摘要:开发了一种有趣的新方法来合成埃文斯的手性助剂,收率很高。反过来,另一种新的立体选择性和有效策略也允许从 Morita-Baylis-Hillman 加合物以 34% 的总产率制备 2-恶唑烷酮衍生物。这种恶唑烷酮对从患有乳腺炎感染的动物身上分离的金黄色葡萄球菌菌株进行了测试。DOI:10.3390/molecules19067429
-
作为产物:描述:参考文献:名称:N-叔丁基磺酰基 (Bus) 保护基团在氨基酸和肽化学中的应用摘要:报道了叔丁基磺酰基 (Bus) 用于临时保护氨基酸和肽的效用。研究了存在其他 N 和 O 保护基团时的相容性和正交性。DOI:10.1055/s-0029-1217996
-
作为试剂:描述:3,5-Dichloro-2-hydroxy-4-[2-(4-methylpiperazin-1-yl)ethoxy]benzoic acid 、 L-苯丙氨酸乙酯盐酸盐 在 L-苯丙氨酸乙酯盐酸盐 作用下, 以93的产率得到N-[3,5-二氯-2-羟基-4-[2-(4-甲基-1-哌嗪基)乙氧基]苯甲酰]-L-苯丙氨酸乙基酯二盐酸参考文献:名称:Amide compounds and use of the same摘要:式(I)的酰胺化合物:其中R为氨基等,A为亚烷基等,X为O、S等,M为芳基等,R1、R2、R3和R4为H、羟基等,R5为H、烷基等,m为0-6的整数,R6为可选取代的芳基等,R7为H、可选取代的烷基等,其药学上可接受的酸盐和含有其作为活性成分的药物。该酰胺化合物在直接或间接涉及炎症的细胞因子,如IL-8、IL-1、IL-6、TNF-α、GM-CSF等方面表现出卓越的抑制作用,并可用于预防和治疗风湿性疾病、痛风性关节炎等。公开号:US06174887B1
文献信息
-
Enantioselective Rhodium-Catalyzed Atom-Economical Macrolactonization作者:Stephanie Ganss、Bernhard BreitDOI:10.1002/anie.201604301日期:2016.8.8coupling of ω‐allenyl‐substituted carboxylic acids. The use of a modified diop ligand, chiral DTBM‐diop, led to high enantioselectivity (up to 93 % ee). The reaction tolerated a large variety of functionalities, including α,β‐unsaturated carboxylic acids and depsipeptides, and provided the desired macrocycles with very high enantio‐ and diastereoselectivity.
-
Virtues of Volatility: A Facile Transesterification Approach to Boronic Acids作者:Stefan P. A. Hinkes、Christian D. P. KleinDOI:10.1021/acs.orglett.9b00584日期:2019.5.3Boronic acids are an increasingly important compound class for many applications, including C–C bond formation reactions, medicinal chemistry, and diagnostics. The deprotection of boronic ester intermediates is frequently a problematic and inefficient step in boronic acid syntheses. We describe an approach that highly facilitates this transformation by leveraging the volatility of methylboronic acid and
-
Palladium-catalyzed carbonylation of benzylic ammonium salts to amides and esters <i>via</i> C–N bond activation作者:Weijie Yu、Shuwu Yang、Fei Xiong、Tianxiang Fan、Yan Feng、Yuanyuan Huang、Junkai Fu、Tao WangDOI:10.1039/c8ob00488a日期:——An efficient palladium-catalyzed carbonylation reaction of readily available quaternary ammonium salts with CO is reported for the first time to afford arylacetamides and arylacetic acid esters via benzylic C–N bond cleavage. This protocol features mild reaction conditions under atmospheric pressure of CO, a redox-neutral process without an additional oxidant, and a broad substrate scope for various
-
Pseudoephedrine-Directed Asymmetric α-Arylation of α-Amino Acid Derivatives作者:Rachel C. Atkinson、Fernando Fernández-Nieto、Josep Mas Roselló、Jonathan ClaydenDOI:10.1002/anie.201502569日期:2015.7.27Available α‐amino acids undergo arylation at their α position in an enantioselective manner on treatment with base of N′‐aryl urea derivatives ligated to pseudoephedrine as a chiral auxiliary. In situ silylation and enolization induces diastereoselective migration of the N′‐aryl group to the α position of the amino acid, followed by ring closure to a hydantoin with concomitant explulsion of the recyclable
-
[EN] 2, 3, 6-TRISUBSTITUTED-4-PYRIMIDONE DERIVATIVES<br/>[FR] DERIVES DE 2,3,6-TRISUBSTITUE 4-PYRIMIDONE申请人:MITSUBISHI PHARMA CORP公开号:WO2004085408A1公开(公告)日:2004-10-07A pyrimidone derivative having tau protein kinase 1 inhibitory activity which is represented by formula (I) or a salt thereof, or a solvate thereof or a hydrate thereof; useful for prventive and/or therapeutic treatment of diseass such as neurodegenerative diseases (e.g. Alzheimer disease); wherein Q represents CH or nitrogen atom; R represents a C1-C12 alkyl group; the ring of Formula (I): represents piperazine ring or piperidine ring; each X independently represents a C1-C8 alkyl group, an optionally partially hydrogenated C6-C10 aryl ring, an indan ring or the like; m represents an integer of 1 to 3; each Y independently represents a halogen atom, a hydroxy group, a cyano group, a C1-C6 alkyl group or the like; n represents an integer of 0 to 8; when X and Y or two Y groups are attached on the same carbon atom, they may combine to each other to form a C2-C6 alkylene group.
表征谱图
-
氢谱1HNMR
-
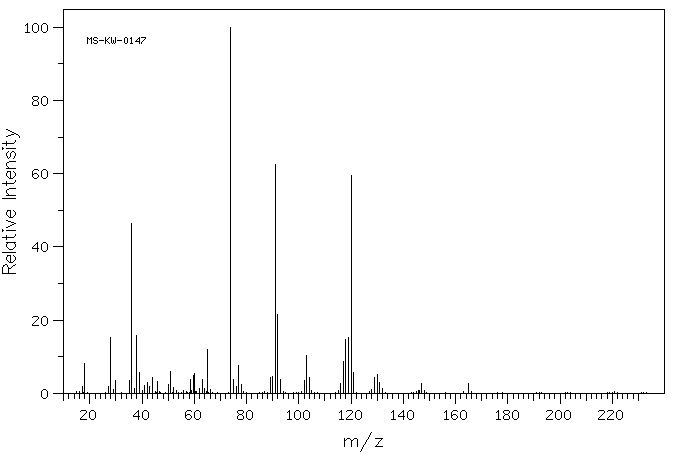
质谱MS
-
碳谱13CNMR
-
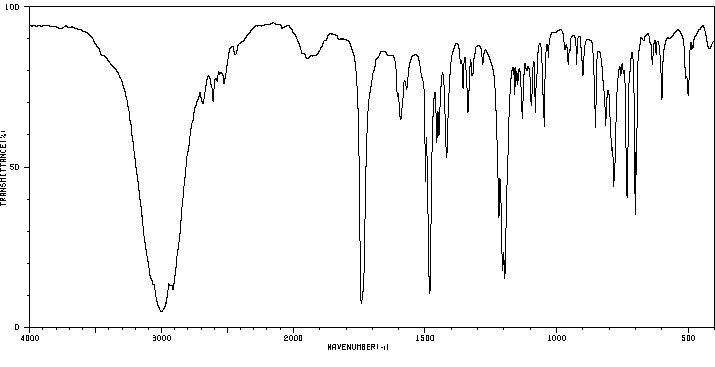
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸