(+/-)-10a-methyl-1,9,10,10a-tetrahydro-(2H)-phenanthren-3-one | 63783-22-2
中文名称
——
中文别名
——
英文名称
(+/-)-10a-methyl-1,9,10,10a-tetrahydro-(2H)-phenanthren-3-one
英文别名
3-Keto-10a-methyl-1,2,3,9,10,10a-hexahydro-phenanthren;10a-Methyl-1,9,10,10a-tetrahydro-2H-phenanthren-3-on;10a-Methyl-1,9,10,10a-tetrahydro-3(2H)-phenanthrenone;10a-methyl-1,2,9,10-tetrahydrophenanthren-3-one
CAS
63783-22-2
化学式
C15H16O
mdl
——
分子量
212.291
InChiKey
GONYVNMFEDYGMU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:16
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
反应信息
-
作为反应物:描述:(+/-)-10a-methyl-1,9,10,10a-tetrahydro-(2H)-phenanthren-3-one 在 palladium on activated charcoal chromium(VI) oxide 、 硫酸 、 氢气 、 对甲苯磺酸 作用下, 以 四氢呋喃 、 乙醚 、 乙醇 、 丙酮 、 甲苯 为溶剂, 25.0 ℃ 、2.0 MPa 条件下, 反应 28.0h, 生成 (4aS,10aS)-10a-Methyl-3-phenyl-1,2,4a,9,10,10a-hexahydro-phenanthrene参考文献:名称:Enthalpy and Entropy of Conjugative Interaction in a Nearly Coplanar Styrene and Cinnamyl Radical摘要:Conjugative interaction in styrenes designed to constrain the dihedral angle between the planes of the phenyl and olefinic groups near to coplanarity (21 degrees by X-ray) is greater by 0.7 kcal mol(-1) than that in a styrene in which the phenyl group is unconstrained (ad libitum) (-35 degrees by MM2 molecular mechanical calculation). Conjugative interaction in a cinnamyl radical similarly constrained to maintain the phenyl and allyl moieties nearly coplanar (15 degrees by MM2) is greater by 1.1 kcal mol(-1) than in a cinnamyl radical in which the phenyl group is ad libitum. Efforts to separate the contribution of pi-electron delocalization from the nonbonded steric factor by employing MM2 calculations proved quantitatively unsatisfactory.DOI:10.1021/ja983088d
-
作为产物:参考文献:名称:A Novel Rearrangement of α,β-Unsaturated Ketones摘要:DOI:10.1246/bcsj.37.151
文献信息
-
Isoe; nakazaki, Chemistry and industry, 1959, p. 1574作者:Isoe、nakazakiDOI:——日期:——
-
Solvolytic rearrangements accompanied by multiple alkyl shifts作者:Howard W. Whitlock、Larry E. OvermanDOI:10.1021/ja00738a026日期:1971.5
表征谱图
-
氢谱1HNMR
-
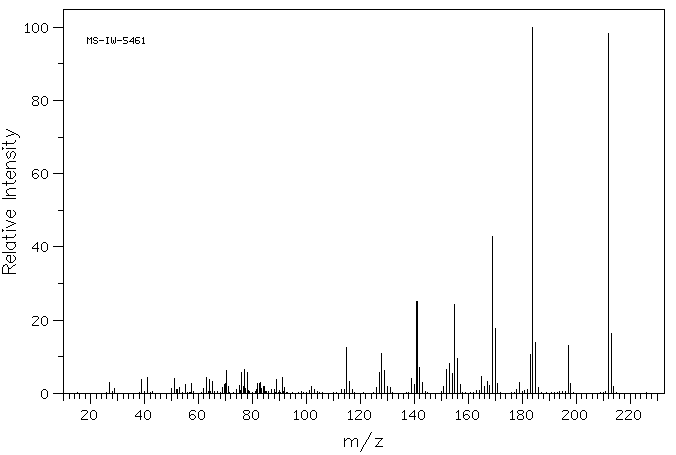
质谱MS
-
碳谱13CNMR
-
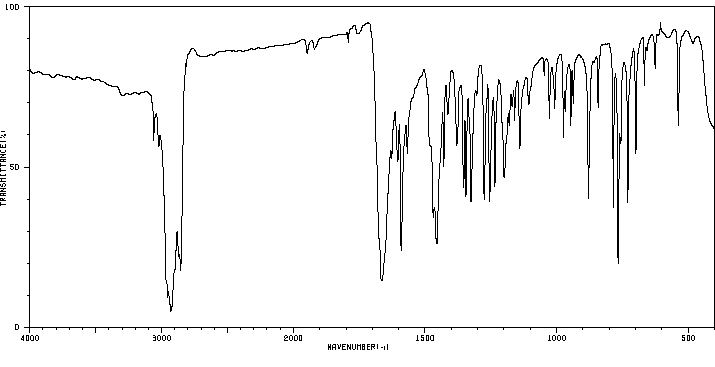
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-2,2'',3,3''-四氢-6,6''-二-9-菲基-1,1''-螺双[1H-茚]-7,7''-二醇
(6,6)-苯基-C61己酸甲酯
高雌二醇
马兜铃酸钠
马兜铃酸盐
马兜铃酸C
马兜铃酸B
马兜铃酸(1:1MIXTUREOFARISTOLOCHICACIDIANDARISTOLOCHICACIDII)
马兜铃酸 Ia
马兜铃酸 IVa
马兜铃酸
颜料黑32
颜料红179
颜料红178
颜料红149
颜料红123
顺式-菲-1,2-二醇-3,4-环氧化物
顺式-苯并(a)屈-11,12-二醇-13,14-环氧化物
雷公藤酚A
镁二(1,4,5,6,7,16,17,18,19,19,20,20-十二氯六环[14.2.1.14,7.02,15.03,8.09,14]二十-5,9,11,13,17-五烯-11-磺酸酯)
钩大青酮
钩大青酮
钙(2+)12-羟基十八烷酸酯
酒石酸布托诺啡
那布扶林
还原红32
足球烯
贝那他汀B
贝母兰素
萘并[2,3-b]荧蒽
萘并[2,1-e][1]苯并二硫杂环戊烷
萘并[2,1-C:7,8-C']二菲
萘并[1,2-e][2]苯并呋喃-1,3-二酮
萘并[1,2-b]屈
萘并[1,2-a]蒽
萘并[1,2-B]菲-6-醇
萘二(六氯环戊二烯)加合物
萘,8-溴-1,2,3-三(1,1-二甲基乙基)-6-甲基-
菲醌单缩氨基硫脲
菲醌
菲并[9,10]呋喃
菲并[9,10-e]醋菲烯
菲并[4,5-bcd]噻吩
菲并[4,5-bcd]呋喃-3-醇
菲并[4,3-d]-1,3-二噁唑-5-羧酸,10-羟基-9-甲氧基-6-硝基-
菲并[3,2-b]噻吩
菲并[2,1-d]噻唑
菲并[2'',1'',10'':4,5,6;7'',8'',9'':4',5',6']二异喹啉并[2,1-a:2',1'-a']二萘嵌间二氮杂苯-8,13-二酮
菲并(3,4-b)噻吩
菲并(1,2-b)噻吩