苯甲酸,2-(4,5-二氢-4,4-二甲基-5-羰基-2-[口噁]唑基)-,甲基酯 | 13059-60-4
中文名称
苯甲酸,2-(4,5-二氢-4,4-二甲基-5-羰基-2-[口噁]唑基)-,甲基酯
中文别名
甘氨酰甘氨酸盐酸盐;盐酸-N-甘氨酰甘氨酸;一缩二氨基乙酸盐酸盐一水合物;盐酸双甘氨肽;盐酸-N-甘氨酰甘氨酸一水合物;双甘氨肽盐酸盐一水合物;盐酸甘胜一水合物;双甘氨肽盐酸盐;盐酸双甘氨肽一水合物
英文名称
glycylglycine hydrochloride
英文别名
glycylglycin hydrochloride;Gly-Gly hydrochloride;2-[(2-Aminoacetyl)amino]acetic acid;hydron;chloride
CAS
13059-60-4;23851-28-7
化学式
C4H8N2O3*ClH
mdl
MFCD00035438
分子量
168.58
InChiKey
YHBAZQDEMYQPJL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:128 - 133°C
-
溶解度:甲醇(微溶)、水(微溶)
计算性质
-
辛醇/水分配系数(LogP):-4.52
-
重原子数:10
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:92.4
-
氢给体数:4
-
氢受体数:4
安全信息
-
WGK Germany:3
-
海关编码:2924199090
SDS
反应信息
-
作为反应物:描述:苯甲酸,2-(4,5-二氢-4,4-二甲基-5-羰基-2-[口噁]唑基)-,甲基酯 在 N-氯-N-甲基-4-甲苯磺酰胺 、 sodium perchlorate 作用下, 以 乙腈 为溶剂, 生成 N-chloroglycylglycine参考文献:名称:Use of N-chloro-N-methyl-p-toluenesulfonamide in N-chlorination reactions摘要:Second-order rate constants (k2) were determined for the addition of ten nitrogenous organic compounds (benzylamine, 2,2,2-trifluoethylamine chlorhidrate, methylamine chlorhidrate, glycine ethyl ester chlorhidrate, glycine, glycylglycine chlorhidrate, morpholine, pyperidine, pyperazine and dimethylamine) to the N-chloro-N-methyl-p-toluenesulfonamide (NCNMPT) in the formation reaction of N-chloramines in aqueous solution at 25 degrees C and ionic strength 0.5M. The series of nucleophiles considered is structurally very varied and covers five pKa units. The kinetic behaviour is similar for all compounds, being the elementary step the transfer of chlorine from the NCNMPT molecule to the nitrogen of the free amino group. These reactions were found first order in both reagents. The values of the rate constants indicate that the more basic amines produce N-chloramines more readily. Rate constants for the nucleophilic attack are shown to correlate with literature data for some of these nitrogenous organic compounds in their reaction with N-methyl-N-nitroso-p-toluenesulfonamide. Both reactions involve that the rate determining step is the attack of nitrogenous compounds upon electrophilic centre (Cl or else NO group). NCNMPT is a particularly interesting substrate, for which has not hitherto been published kinetic information, that allows us to assess the efficiency and the competitiveness of this reaction and compare it with other agents with a Cl+ atom. Copyright (c) 2013 John Wiley & Sons, Ltd.DOI:10.1002/poc.3127
-
作为产物:参考文献:名称:新型酰胺衍生物的合成及止血活性摘要:通过三步法合成了八种含有抗纤维蛋白溶解剂(氨甲环酸、氨基己酸、4-(氨基甲基)苯甲酸和甘氨酸——天然氨基酸)的二肽,收率良好或非常好。DMT/NMM/TsO - (4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-甲基吗啉鎓甲苯-4-磺酸盐)用作偶联剂。溶血试验用于研究二肽对血液成分的影响。血浆凝固试验用于检查它们对凝血酶时间 (TT)、凝血酶原时间 (PT) 和活化部分促凝血酶原激酶时间 (aPTT) 的影响。溶血水平不超过1%。在凝血测试中,TT、PT 和 aPTT 没有区分任何化合物。所有酰胺1 – 8的凝血酶原时间是相似的。在酰胺1-4和8存在下获得的结果略低于其他化合物和阳性对照,并且与 TA 获得的结果相似。在酰胺3的情况下,观察到显着降低的 aPTT。用酰胺3和 TA 处理的血浆观察到的 aPTT 具有可比性。在酰胺6和8的情况下, 发现 TT 值DOI:10.3390/molecules27072271
文献信息
-
Studies relating to the ferredoxins. Part 3. The synthesis of some cysteine–glycine peptides for iron–sulphur complexing studies作者:Ambikaipakan Balasubramaniam、Roger J. Burt、George Christou、Brian Ridge、H. N. RydonDOI:10.1039/p19810000310日期:——Three peptides with the general structure Ac-Gly2.(Cys.Glym)n.Cys.Gly3.Cys.Gly2.NH2(1; m= 2 or 3; n= 0 or 1), four with the general structure Ac.Gly2.Cysn.Gly2.NH2(2; n= 1–4), and three with the general structure HS.CH2.CH2.CO.Gly2.(Cys.Gly2)n.NH.CH2.CH2.SH (3; n= 0–2) have been synthesised for iron-sulphur complexing studies.
-
Photochemically Induced Electron Transfer (PET) Catalyzed Radical Cyclization: A Practical Method for Inducing Structural Changes in Peptides by Formation of Cyclic Amino Acid Derivatives作者:Marco Jonas、Siegfried Blechert、Eberhard SteckhanDOI:10.1021/jo010144b日期:2001.10.1A new radical cyclization reaction of unsaturated amino acid derivatives is presented. The reaction is induced by photoelectron transfer (PET) catalysis and proceeds, in comparison to commonly applied methods, under mild, nonoxidizing, and nontoxic conditions in neutral medium. This type of radical cyclization reaction can be used in peptide chemistry for inducing structural changes in peptides.
-
Synthesis and structural characterization of amino acid and peptide derivatives featuring N-(p-bromobenzoyl) substituents as promising connection unit for bio-inspired hybrid compounds作者:Frank Eißmann、Edwin WeberDOI:10.1016/j.molstruc.2011.03.058日期:2011.5Abstract Using a sequence of activation, blocking and peptide coupling procedures, a series of ten amino acid and peptide derivatives 1–6 (a, b, respectively) featuring a p-bromobenzoyl substituent attached to the amino group of the parent compound has been synthesized. X-ray crystal structures of two corresponding amino acid (1a, 1b), two amino acid ester (3a, 3b) and three peptide ester derivatives
-
Synthesis of new lipophilic phosphine oxide derivatives of natural amino acids and their membrane transport properties toward carboxylic acids作者:S. A. Koshkin、A. R. Garifzyanov、N. V. Davletshina、O. N. Kataeva、D. R. Islamov、R. A. CherkasovDOI:10.1134/s1070428015090031日期:2015.9One-pot procedures were developed for the synthesis of lipophilic N-(dialkylphosphorylmethyl) derivatives of natural amino acids with high yields from dioctyl- or didecylphosphine oxide, formaldehyde, and amino acid in the presence of amino acid hydrochloride. The reactions with some amino acids were also effective under catalysis by crown ether. The structure of the isolated N-(dialkylphosphorylmethyl)开发了一锅法,用于在氨基酸盐酸盐的存在下,从二辛基或二癸基氧化膦,甲醛和氨基酸以高收率合成天然氨基酸的亲脂性N-(二烷基磷酰基甲基)衍生物。与某些氨基酸的反应在冠醚的催化下也是有效的。分离的N-(二烷基磷酰基甲基)和N,N-双(二烷基磷酰基甲基)氨基酸的结构是根据1 H,13 C和31 P NMR和质谱确定的。(S)-N的结构通过X射线分析证实了-[[(二环己基磷酰基)甲基]-α-丙氨酸,并表征了其分子在晶体中的分子间缔合。研究了新型磷酸化氨基酸相对于多官能团羧酸的膜传输特性,并估算了影响酸性底物膜传输效率和选择性的因素。揭示了通过含有N,N-双[(二辛基磷酰基)甲基]-β-丙氨酸的液膜选择性提取戊二酸。
-
The α-Effect in S<sub>N</sub>Ar Reaction of Y-Substituted-Phenoxy-2,4-Dinitrobenzenes with Amines: Reaction Mechanism and Origin of the α-Effect作者:Hyo-Jin Cho、Min-Young Kim、Ik-Hwan UmDOI:10.5012/bkcs.2014.35.8.2448日期:2014.8.20The α-Effect in SNAr Reaction of Aryloxy-2,4-dinitrobenzenes Bull. Korean Chem. Soc. 2014, Vol. 35, No. 8 2449stituted-phenoxy-2,4-dinitrobenzenes (1a-1g) with hydra-zine and glycylglycine as an α-nucleophile and a referencenormal-nucleophile, respectively to obtained further infor-mation on the origin of the α-effect in the S
表征谱图
-
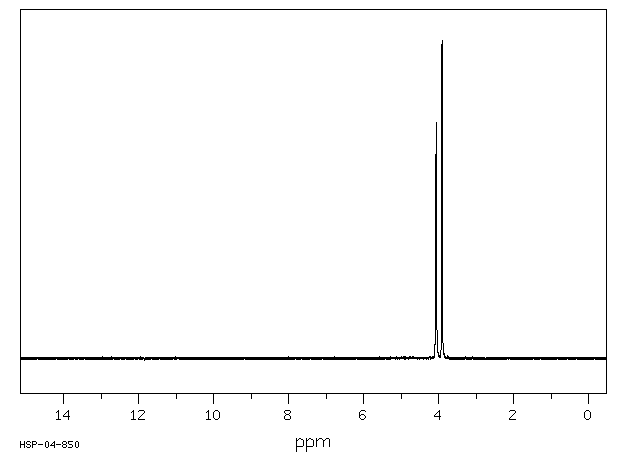
氢谱1HNMR
-
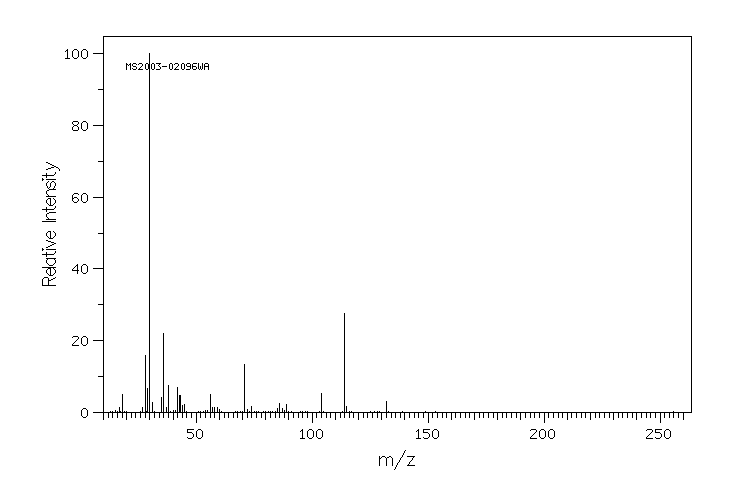
质谱MS
-
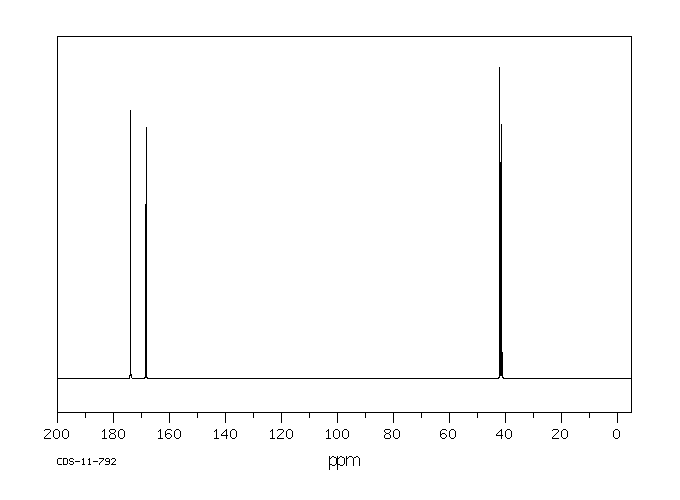
碳谱13CNMR
-
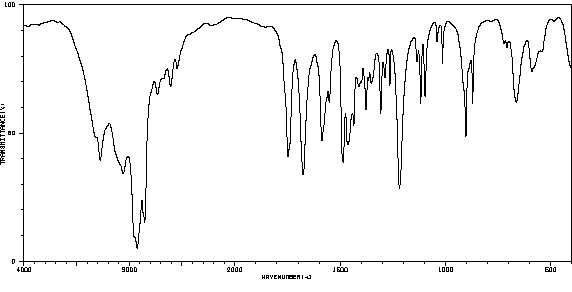
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸