2,6-dimethyl-3-phenyl-4H-pyran-4-one | 40122-80-3
中文名称
——
中文别名
——
英文名称
2,6-dimethyl-3-phenyl-4H-pyran-4-one
英文别名
2,6-Dimethyl-3-phenylpyran-4-one
CAS
40122-80-3
化学式
C13H12O2
mdl
——
分子量
200.237
InChiKey
RMDLFJOOIBPSAU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:15
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.15
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
反应信息
-
作为反应物:描述:2,6-dimethyl-3-phenyl-4H-pyran-4-one 在 ammonium hydroxide 、 溴 、 溶剂黄146 、 三氯氧磷 作用下, 以 乙醇 为溶剂, 反应 74.0h, 生成 3-bromo-4-chloro-2,6-dimethyl-5-phenylpyridine参考文献:名称:A Suzuki-Miyaura Approach to a Series of Forensically Relevant Pyridines摘要:本文介绍了制备十六种具有重要法医意义的 3,5 二取代 2,4- 和 2,6 二甲基吡啶的简便通用方法。一系列环取代的苯硼酸与 3,5-二溴-2,4-二甲基吡啶和 3,5-二溴-2,6-二甲基吡啶之间的铃木交叉偶联反应被用作关键步骤。DOI:10.1055/s-2006-942535
-
作为产物:描述:参考文献:名称:4-吡啶酮作为潜在抗疟药的合成与构效关系。摘要:已鉴定出一系列二芳基醚取代的4-吡啶酮,其在体外和体内对鼠恶性疟原虫的有效抗疟活性优于氯喹对恶性疟原虫的抗疟活性。这些是通过对侧链变化对活性的影响进行系统研究而从抗球虫药clopidol衍生而来的。相对于氯吡咯醇,最具活性的化合物在体外抑制恶性疟原虫的IC50改善了500倍以上,而在小鼠中相对于针对约氏疟原虫的ED50则提高了约100倍。这些化合物已在其他地方显示出通过抑制细胞色素bc1络合物处线粒体电子传递的作用而选择性发挥作用。DOI:10.1021/jm0705760
文献信息
-
Heterocyclic compounds申请人:THE WELLCOME FOUNDATION LIMITED公开号:EP0447164A1公开(公告)日:1991-09-18A compound of formula (I):- wherein R¹ represents a hydrogen or halogen atom, or a cyano group; R² represents an optionally substituted carbocyclic group having 6 to 10 ring atoms and containing at least one aromatic ring; an optionally substituted heterocyclic group having 5 to 10 ring atoms, including 1 to 4 heteroatoms selected from O,N and S, and containing at least one aromatic ring; or an optionally substituted C₃₋₆cycloalkyl, C₃₋₆cycloalkyl- C₁₋₆alkyl, or C₁₋₁₀alkyl group; R³ and R⁴, which may be the same or different, each represent a hydrogen or halogen atom, or a C₁₋₆alkyl group optionally substituted by 1 to 3 halogen atoms; and R⁵ represents a hydrogen atom, a hydroxyl group, or a C₁₋₆alkyl group, optionally substituted by hydroxy, carboxy, amino or mono- or di-(C₁₋₄)alkyl amino, and salts and other physiologically functional derivative thereof. The compounds are useful in the treatment of parasitic infections eg. malaria, coccidiosis and Pneumocystis carinii pneumonia.化合物的化学式为(I):其中R¹代表氢原子或卤素原子或氰基;R²代表可选取代的碳环基团,其有6至10个环原子并含有至少一个芳香环;可选取代的杂环基团,其有5至10个环原子,包括1至4个从O、N和S中选取的杂原子,并含有至少一个芳香环;或可选取代的C₃₋₆环烷基、C₃₋₆环烷基- C₁₋₆烷基,或C₁₋₁₀烷基;R³和R⁴,它们可以相同也可以不同,每个代表氢原子或卤素原子,或由1至3个卤素原子取代的C₁₋₆烷基;R⁵代表氢原子、羟基,或由羟基、羧基、氨基或单烷基或双烷基(C₁₋₄)氨基取代的C₁₋₆烷基,以及其盐和其他生理功能衍生物。这些化合物在治疗寄生虫感染,例如疟疾、球虫病和肺孢子虫肺炎中有用。
-
Pyridine derivatives申请人:ZENECA LIMITED公开号:EP0453210A2公开(公告)日:1991-10-23The invention concerns pharmaceutically useful novel compounds of the formula I, and their non-toxic salts, and pharmaceutical compositions containing them. The novel compounds are of value in treating conditions such as hypertension and congestive heart failure. The invention further concerns processes for the manufacture of the novel compounds and the use of the compounds in medical treatment.本发明涉及在医药上有用的式 I 新型化合物及其无毒盐,以及含有它们的药物组合物。这些新型化合物具有治疗高血压和充血性心力衰竭等疾病的价值。本发明还涉及新型化合物的生产工艺以及这些化合物在医疗中的用途。
-
New nonpeptide angiotensin II receptor antagonists. 3. Synthesis, biological properties, and structure-activity relationships of 2-alkyl-4-(biphenylylmethoxy)pyridine derivatives作者:Robert H. Bradbury、Christopher P. Allott、Michael Dennis、J. Alan Girdwood、Peter W. Kenny、John S. Major、Alec A. Oldham、Arnold H. Ratcliffe、Janet E. RivettDOI:10.1021/jm00061a016日期:1993.4A novel series of nonpeptide angiotensin II (AII) receptor antagonists is reported, derived from linkage of the biphenylyltetrazole moiety found in previously described antagonists via a methyleneoxy chain to the 4-position of a 3-substituted 2,6-dialkylpyridine. When evaluated in an in vitro binding assay using a guinea pig adrenal membrane preparation, compounds in this series generally gave IC50 values in the range 0.005-0.5 muM. A variety of substituents was found to be effective at the 3-position of the pyridine ring. On intravenous administration in a normotensive rat model, the more potent compounds inhibited the AII-induced pressor response with ED50 values in the range 0.1-1.0 mg/kg. One of the compounds, 2-ethyl-5,6,7,8-tetrahydro-4-[2'-(1H-tetrazol-5-yl)biphenyl-4-yl]methoxy}quinoline (26), demonstrated good oral activity in two rat models. At doses in the range 1-10 mg/kg po in AII-infused, conscious, normotensive rats, the compound exhibited a dose-related inhibition of the pressor response with a good duration of action at the higher doses. In a renal hypertensive rat model compound 26 showed a rapid and sustained lowering of blood pressure at a dose of 5 mg/kg po. Based on its profile, this compound, designated ICI D6888, has been selected for evaluation in volunteers.
-
US5130318A申请人:——公开号:US5130318A公开(公告)日:1992-07-14
-
US5198439A申请人:——公开号:US5198439A公开(公告)日:1993-03-30
表征谱图
-
氢谱1HNMR
-
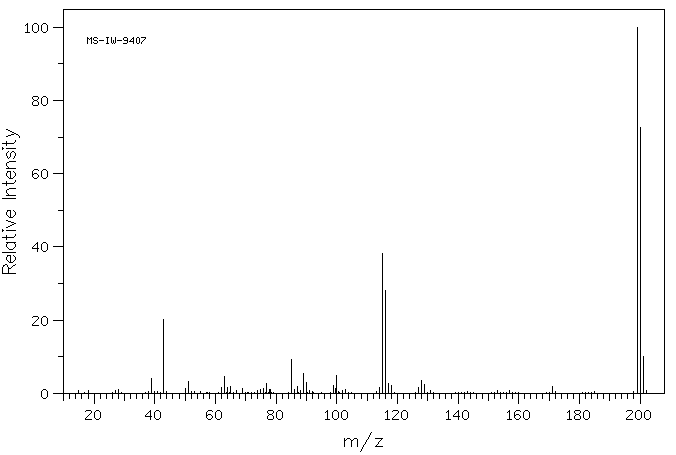
质谱MS
-
碳谱13CNMR
-
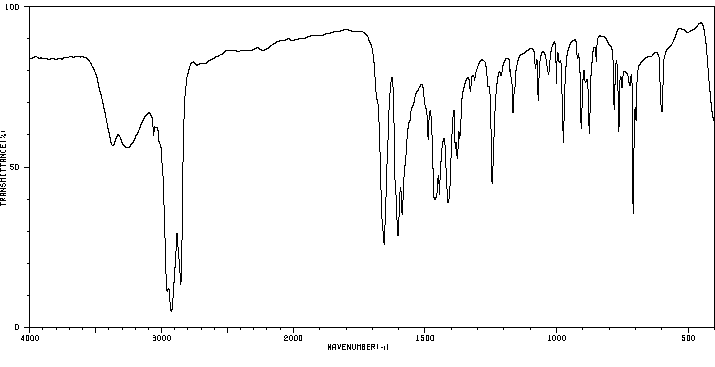
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2R)-2,6-二羟基-5-[(E)-丙-1-烯基]-1,2-二氢吡喃并[3,2-b]吡咯-3,7-二酮
黄绿青霉素
麦芽醇
麦芽酚铁
马索亚内酯
香豆酸
香豆灵酸甲酯
香叶吡喃
顺式-1-(3-呋喃基)-1,7,8,8a-四氢-5,8a-二甲基-3H-2-苯并吡喃-3-酮
靠曼酸乙酯; 4-吡喃酮-2-羧酸乙酯
靠曼酸
镭杂9蛋白质
铝3-羟基-2-甲基-4-吡喃酮
钠[(1E,7E,9E,11E)-6-羟基-1-(3-羟基-6-氧代-2,3-二氢吡喃-2-基)-5-甲基十七碳-1,7,9,11-四烯-4-基]硫酸盐
避虫酮
辛伐他汀杂质C
褐鸡蛋花素
脱氢乙酸缩氨基硫脲
脱氢乙酸
罌粟酸
维达列汀
福司曲星
福司曲星
磷内酯霉素F
磷内酯霉素E
磷内酯霉素D
磷内酯霉素A
白屈菜酸
甲基6-甲氧基-2-甲基-5-氧代四氢-2H-吡喃-2-羧酸酯
甲基6-氧杂双环[3.1.0]己烷-1-羧酸酯
甲基4-氧代-4H-吡喃-3-羧酸酯
甲基4,6-二-O-乙酰基-2,3-二脱氧己-2-烯基吡喃糖苷
甲基2H-吡喃-5-羧酸酯
甲基2-乙氧基-6-甲基-3,4-二氢-2H-吡喃-4-羧酸酯
甲基2-乙氧基-4-氧代-3,4-二氢-2H-吡喃-5-羧酸酯
甲基2-乙氧基-3-甲基-4-氧代-3,4-二氢-2H-吡喃-5-羧酸酯
甲基(4S)-2-氧代-4-[(2E)-1-氧代-2-丁烯-2-基]-3,4-二氢-2H-吡喃-5-羧酸酯
甲基(2S,5R)-5-甲氧基-3-硝基-2,5-二氢-2-呋喃羧酸酯
甲基(2S)-4-甲基-3,6-二氢-2H-吡喃-2-羧酸酯
甲基(2R)-四氢-2H-吡喃-2-羧酸酯
环庚三烯并[b]吡喃-2(5H)-酮,9-(3-丁烯基)-3-(环丙基苯基甲基)-6,7,8,9-四氢-4-羟基-
环吡酮杂质B
焦袂康酸O-甲基醚
沉香四醇
氨甲酸,[3-[(苯基甲基)氨基]三环[3.3.1.13,7]癸-1-基]-,1,1-二甲基乙基酯(9CI)
毛子草酮
棒曲霉素-13C3
棒曲霉素
木菌素
木糖酸二钠盐