2-(2,2-dimethyl-[1,3]dioxan-5-ylidene)-butyric acid ethyl ester | 496947-02-5
中文名称
——
中文别名
——
英文名称
2-(2,2-dimethyl-[1,3]dioxan-5-ylidene)-butyric acid ethyl ester
英文别名
Ethyl 2-(2,2-dimethyl-1,3-dioxan-5-ylidene)butanoate
CAS
496947-02-5
化学式
C12H20O4
mdl
——
分子量
228.288
InChiKey
YTWGRFGGVKRQAZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:16
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:44.8
-
氢给体数:0
-
氢受体数:4
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3-ethyl-4-hydroxymethyl-5H-furan-2-one 496947-06-9 C7H10O3 142.155
反应信息
-
作为反应物:描述:2-(2,2-dimethyl-[1,3]dioxan-5-ylidene)-butyric acid ethyl ester 在 palladium on activated charcoal 盐酸 、 氢气 作用下, 以 甲醇 、 乙醇 为溶剂, 反应 19.17h, 生成 3-ethyl-4-methyl-2(5H)-furanone参考文献:名称:Selective Catalytic Hydrogenations and Hydrogenolyses VIII [1]: Stereoselective Synthesis of the Stereomeric Pilopyl Alcohols摘要:The stereoselective synthesis of pilopyl- and isopilopyl alcohol is reported. The reaction of dimethyldioxanone and diethoxyphosphoryl-butyric acid ethyl ester afforded the corresponding dioxanylidenbutyric acid ester as the key intermediate. Upon treatment with mineral acid it cyclized giving 3-ethyl-4-hydroxymethylfuran-2-one which in turn could be converted either to 3-ethyl-4-methylfuranone or pilopyl alcohol with excellent stereoselectivity and quantitative chemical yield. On the other hand, hydrogenation and subsequent cyclization of the same key compound furnished isopilopyl alcohol with good stereomeric purity and yield.DOI:10.1007/s00706-002-0484-9
-
作为产物:描述:2-膦酰丁酸三乙脂 、 2,2-二甲基-1,3-二恶烷-5-酮 在 sodium hydride 作用下, 以 四氢呋喃 为溶剂, 反应 1.5h, 生成 2-(2,2-dimethyl-[1,3]dioxan-5-ylidene)-butyric acid ethyl ester参考文献:名称:三和四取代的α,α'-链烯二醇的非酶几何选择性酰化摘要:已经开发出三和四取代的2-亚烷基-1,3-丙二醇的高度几何选择性有机催化酰化。各种四取代的2-亚烷基-1,3-丙二醇的高度E-选择性酰化是通过非酶法首次以96%至> 99%的选择性实现的。DOI:10.1002/adsc.201200242
表征谱图
-
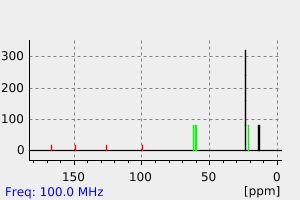
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯