环香豆素 | 518-20-7
中文名称
环香豆素
中文别名
吡喃香豆素
英文名称
cyclocoumarol
英文别名
pyranocoumarin;2-methoxy-2-methyl-4-phenyl-3,4-dihydro-2H-pyrano[3,2-c]chromen-5-one;2-Methoxy-2-methyl-4-phenyl-3,4-dihydro-2H-pyrano[3,2-c]chromen-5-on;Cyclocumarol;2-methoxy-2-methyl-4-phenyl-3,4-dihydropyrano[3,2-c]chromen-5-one
CAS
518-20-7
化学式
C20H18O4
mdl
——
分子量
322.361
InChiKey
ZGFASEKBKWVCGP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:166°
-
沸点:488.6±45.0 °C(Predicted)
-
密度:1.27±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.7
-
重原子数:24
-
可旋转键数:2
-
环数:4.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:44.8
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险等级:6.1(b)
-
危险品标志:T+
-
安全说明:S28,S36/37,S45
-
危险类别码:R27
-
WGK Germany:3
-
海关编码:2932999099
-
包装等级:III
-
危险类别:6.1(b)
-
危险品运输编号:UN 2811
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— warfarin 54288-86-7 C19H16O4 308.334 外消旋-2-甲基-4-苯基-4H-吡喃并[3,2-c]苯并吡喃-5-酮 2-methyl-4-phenylpyrano[3,2-c]chromen-5(4H)-one 15151-14-1 C19H14O3 290.318
反应信息
-
作为反应物:参考文献:名称:华法林及其酚类代谢产物的高产率合成:新化合物。摘要:DOI:10.1002/jps.2600720732
-
作为产物:参考文献:名称:Studies on 4-Hydroxycoumarins. V. The Condensation of α,β-Unsaturated Ketones with 4-Hydroxycoumarin1摘要:DOI:10.1021/ja01234a019
文献信息
-
CHELATE NANOEMULSION FOR MRI申请人:Port Marc公开号:US20130309176A1公开(公告)日:2013-11-21The present invention relates to an oil-in-water nanoemulsion composition for MRI, comprising: an aqueous phase, representing 70% to 90% by weight of the composition, advantageously 75% to 85% and more advantageously from 78% to 82% a lipid phase comprising an oil, representing 9.5% to 29.5% by weight of the composition, advantageously 14% to 25% and more advantageously 17% to 21%, a surfactant at the interface between the aqueous and lipid phases, the surfactant comprising at least one amphiphilic paramagnetic metal chelate and optionally an amphiphilic lipid; the total content of surfactant by weight relative to the oil being between 4% and 10% and advantageously between 5% and 8%; the total content of surfactant by weight relative to the composition being between 0.35% and 2.95% and advantageously between 0.5% and 2%; the oil comprising at least 70%, advantageously at least 80%, advantageously at least 95% by weight and especially at least 97% of saturated C6-C18, advantageously C6-C14 and more advantageously C6-C10 fatty acids.本发明涉及一种用于磁共振成像的油包水纳米乳液组合物,包括: 水相,表示组合物重量的70%至90%,优选75%至85%,更优选78%至82%; 脂相包括一种油,表示组合物重量的9.5%至29.5%,优选14%至25%,更优选17%至21%; 表面活性剂位于水相和脂相之间的界面上,表面活性剂包括至少一种两性磁性金属螯合物和可选的两性脂质; 相对于油的重量,表面活性剂的总含量在4%至10%之间,优选在5%至8%之间; 相对于组合物的重量,表面活性剂的总含量在0.35%至2.95%之间,优选在0.5%至2%之间; 油包括至少70%,优选至少80%,优选至少95%的饱和C6-C18,优选C6-C14,更优选C6-C10脂肪酸。
-
4-SUBSTITUTED COUMARIN DERIVATIVES AND PREPARATION METHODS AND USES THEREOF申请人:CHEN Lijuan公开号:US20180282315A1公开(公告)日:2018-10-04The present invention pertains to the field of chemical medicine, particularly to 4-substituted coumarin derivatives and preparation methods and applications thereof. The invention provides 4-substituted coumarin derivatives with a structural formula as shown in Formula I. The invention also provides preparation methods and applications for the above 4-substituted coumarin derivatives. The compounds provided in the invention have strong anti-tumor activity with IC50 for plural tumor cell lines between 0.01-5 nM, and it also performs better to inhibit microtubule polymerization and has diversified biological activities and low toxicity, providing new options for drug-sensitive and drug-resistant tumor cells.
-
[EN] DIGLYCIDIC ETHER DERIVATIVE THERAPEUTICS AND METHODS FOR THEIR USE<br/>[FR] PRODUITS THÉRAPEUTIQUES DÉRIVÉS D'ÉTHERS DIGLYCIDIQUES ET LEURS PROCÉDÉS D'UTILISATION申请人:BRITISH COLUMBIA CANCER AGENCY公开号:WO2010000066A1公开(公告)日:2010-01-07This invention provides compound having a structure of Formula I or Formula II. Uses of such compounds for treatment of various indications, including prostate cancer as well as methods of treatment involving such compounds are also provided.这项发明提供了具有化学结构为式I或式II的化合物。还提供了这些化合物用于治疗各种适应症,包括前列腺癌的用途,以及涉及这些化合物的治疗方法。
-
Vectorised Magnetic Emulsion申请人:GUERBET公开号:US20150320889A1公开(公告)日:2015-11-12An oil-in-water nanoemulsion composition for MRI, comprising an aqueous phase, a lipid phase as nanodroplets comprising an oil and magnetic particles based on an iron compound and covered with one or several C8-C22 fatty acids, and a mixture of surfactants at the interface between the aqueous and lipid phases, the mixture of surfactants comprising at least one amphiphilic lipid and at least one amphiphilic targeting ligand.
-
[EN] INHIBITORS OF GLUCOSE-6-PHOSPHATE DEHYDROGENASE FOR TREATING CARDIOVASCULAR AND PULMONARY CONDITIONS<br/>[FR] INHIBITEURS DE LA GLUCOSE-6-PHOSPHATE DÉSHYDROGÉNASE PERMETTANT DE TRAITER DES AFFECTIONS CARDIOVASCULAIRES ET PULMONAIRES申请人:GUPTE SACHIN A公开号:WO2018093856A1公开(公告)日:2018-05-24The present disclosure provides for methods of treating or preventing a cardiovascular disorder and/or a related pulmonary disorder in a subject. In certain embodiments, the method comprises administering a therapeutically effective amount of an inhibitor of Glucose-6-phosphate dehydrogenase (G6PD), or a pharmaceutically acceptable salt, non-salt amorphous form, solvate, poly-morph, tautomer or prodrug thereof.
表征谱图
-
氢谱1HNMR
-
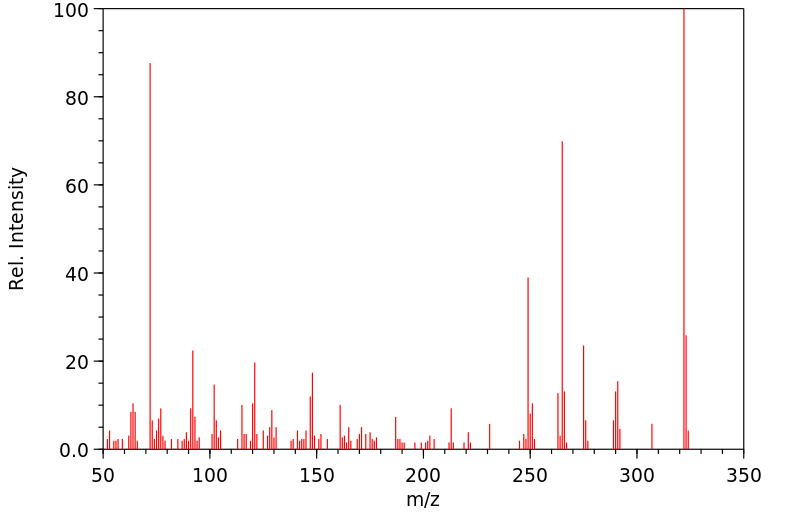
质谱MS
-
碳谱13CNMR
-
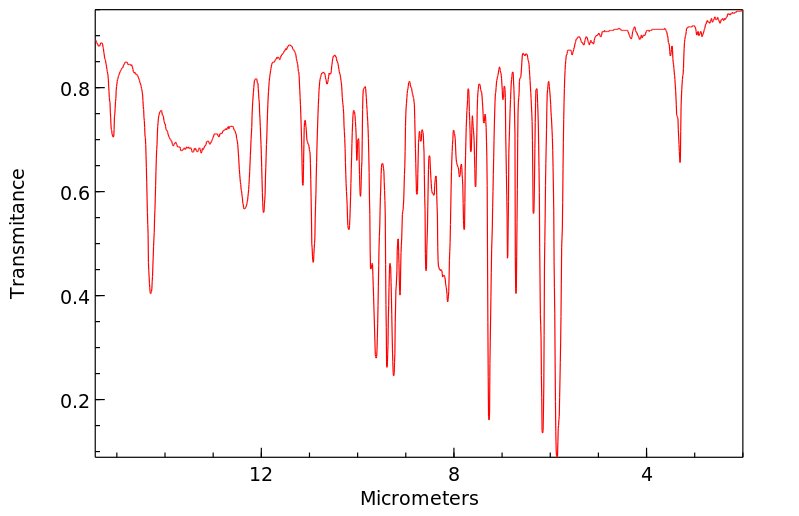
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄皮香豆精
黄木亭
黄曲霉素P2
黄曲霉素P1
黄曲霉素G2-13C17-同位素
黄曲霉素G2
黄曲霉素G1-13C17-同位素
黄曲霉素B2-13C17-同位素
黄曲霉素B1-13C17-同位素
黄曲霉素B1 8,9-环氧化物
黄曲霉素 G1
黄曲霉毒醇Ⅱ
黄曲霉毒醇M1
黄曲霉毒醇A
黄曲霉毒素M2
黄曲霉毒素M1-(O-羧甲基)肟
黄曲霉毒素G2a
黄曲霉毒素G19,10-环氧化物
黄曲霉毒素B2
黄曲霉毒素B1二氯化物
黄曲霉毒素B1-8,9-二氯化物
黄曲霉毒素B1-(O-羧甲基)肟
黄曲霉毒素 Q1
黄曲霉毒素 M1
黄曲霉毒素 B2
黄曲霉毒素 B1
黄曲霉毒素
香豆霉素
香豆素6H
香豆素545T
香豆素545
香豆素525
香豆素343甲酯
香豆素338
香豆素314T
香豆素175
香豆素152
香豆素106
香豆素-D4
香豆素-6-磺酰氯
香豆素-6-甲醛
香豆素-5-氧丁酸
香豆素-4-乙酸
香豆素-3腈
香豆素-35
香豆素-3-羧酸酸酐
香豆素-3-羧酸琥珀酰亚胺酯
香豆素-3-羧酸乙酯
香豆素-3-羧酸
香豆素-3-甲酰氯