8-(3-甲氧基-苯基)-1,4-二氧杂-螺[4.5]癸烷-8-甲腈 | 13225-35-9
中文名称
8-(3-甲氧基-苯基)-1,4-二氧杂-螺[4.5]癸烷-8-甲腈
中文别名
——
英文名称
1-(3-Methoxy-phenyl)-4-oxo-cyclohexan-1-carbonitril-ethylenketal
英文别名
8-(3-methoxy-phenyl)-1,4-dioxa-spiro[4.5]decane-8-carbonitrile;4-(m-anisyl)-4-cyanocyclohexanone, ethylene ketal;4-(3-methoxyphenyl)-4-cyano-1,1,ethylenedioxy-cyclohexane;8-(m-Methoxyphenyl)-1,4-dioxaspiro(4.5)decane-8-carbonitrile;8-(3-methoxyphenyl)-1,4-dioxaspiro[4.5]decane-8-carbonitrile
CAS
13225-35-9
化学式
C16H19NO3
mdl
——
分子量
273.332
InChiKey
RQDGRMRKELEEJF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:70-72 °C
-
沸点:451.9±45.0 °C(Predicted)
-
密度:1.19±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:20
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.56
-
拓扑面积:51.5
-
氢给体数:0
-
氢受体数:4
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 4-cyano-4-(m-methoxyphenyl)cyclohexanone 13225-34-8 C14H15NO2 229.279 —— Methyl 5-cyano-5-(m-methoxyphenyl)-2-oxocyclohexanecarboxylate 13225-33-7 C16H17NO4 287.315 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 8-(3-methoxy-phenyl)-1,4-dioxa-spiro[4.5]decane-8-carboxylic acid amide 56231-45-9 C16H21NO4 291.347 —— 8-(3-methoxyphenyl)-N-methyl-1,4-dioxaspiro[4.5]decan-8-amine 79741-31-4 C16H23NO3 277.364 —— 4-(m-anisyl)-4-isocyanatocyclohexanone, ethylene ketal 65618-95-3 C16H19NO4 289.331
反应信息
-
作为反应物:描述:8-(3-甲氧基-苯基)-1,4-二氧杂-螺[4.5]癸烷-8-甲腈 在 sodium hydroxide 、 lithium aluminium tetrahydride 、 叠氮磷酸二苯酯 、 三乙胺 作用下, 以 四氢呋喃 、 乙二醇 为溶剂, 反应 30.0h, 生成 8-(3-methoxyphenyl)-N-methyl-1,4-dioxaspiro[4.5]decan-8-amine参考文献:名称:4-Amino-4-arylcyclohexanones and their derivatives, a novel class of analgesics. 1. Modification of the aryl ring摘要:Investigation of central nervous system activity of phenylcyclohexylamines was continued by preparation of "reversed" analogues. Following the unexpected finding of analgesic activity with 1-(dimethylamino)-1-phenylcyclohexylamine, the SAR of the series was investigated. Synthesis starts by double Michael reaction of acrylate on arylacetonitriles. Following cyclization, decarboxylation, ketalization, and saponification, the geminally substituted acid is rearranged to the isocyanate by means of (C6H5O)2PON3. Isocyanates were then converted to the title compounds. Analgesic activity is very sensitive to the nature and position of the substitutent on the aromatic ring. The most potent compounds in this series (p-CH3, p-Br) showed 50% the potency of morphine. Deletion of the ring oxygen abolishes activity.DOI:10.1021/jm00178a014
-
作为产物:描述:Methyl 5-cyano-5-(m-methoxyphenyl)-2-oxocyclohexanecarboxylate 在 硫酸 、 对甲苯磺酸 作用下, 以 溶剂黄146 、 苯 为溶剂, 反应 30.0h, 生成 8-(3-甲氧基-苯基)-1,4-二氧杂-螺[4.5]癸烷-8-甲腈参考文献:名称:4-Amino-4-arylcyclohexanones and their derivatives, a novel class of analgesics. 1. Modification of the aryl ring摘要:Investigation of central nervous system activity of phenylcyclohexylamines was continued by preparation of "reversed" analogues. Following the unexpected finding of analgesic activity with 1-(dimethylamino)-1-phenylcyclohexylamine, the SAR of the series was investigated. Synthesis starts by double Michael reaction of acrylate on arylacetonitriles. Following cyclization, decarboxylation, ketalization, and saponification, the geminally substituted acid is rearranged to the isocyanate by means of (C6H5O)2PON3. Isocyanates were then converted to the title compounds. Analgesic activity is very sensitive to the nature and position of the substitutent on the aromatic ring. The most potent compounds in this series (p-CH3, p-Br) showed 50% the potency of morphine. Deletion of the ring oxygen abolishes activity.DOI:10.1021/jm00178a014
文献信息
-
Novel azabicyclo octane derivative and process for preparing same申请人:Tanabe Seiyaku Co., Ltd公开号:US03932429A1公开(公告)日:1976-01-13A compound of the formula: ##SPC1## Wherein R.sup.1 is hydrogen, phenyl, alkyl of one to five carbon atoms, cycloalkyl of three to four carbon atoms, or alkyl of one to five carbon atoms having a substituent selected from the group consisting of phenyl, benzoyl and 4-fluorobenzoyl, R.sup.2 is hydrogen or alkyl of one to three carbon atoms, and R.sup.3 is hydrogen, alkanoyl of two to six carbon atoms, benzoyl or nicotinoyl, is disclosed. Several methods for preparing the compound (I) is also disclosed. The compound (I) and a pharmaceutically acceptable acid addition salt thereof are useful as analgesic agents.
-
LEDNICER D.; VOIGTLANDER P. F.; EMMERT D. E., J. MED. CHEM., 1980, 23, NO 4, 424-430作者:LEDNICER D.、 VOIGTLANDER P. F.、 EMMERT D. E.DOI:——日期:——
-
US3932429A申请人:——公开号:US3932429A公开(公告)日:1976-01-13
表征谱图
-
氢谱1HNMR
-
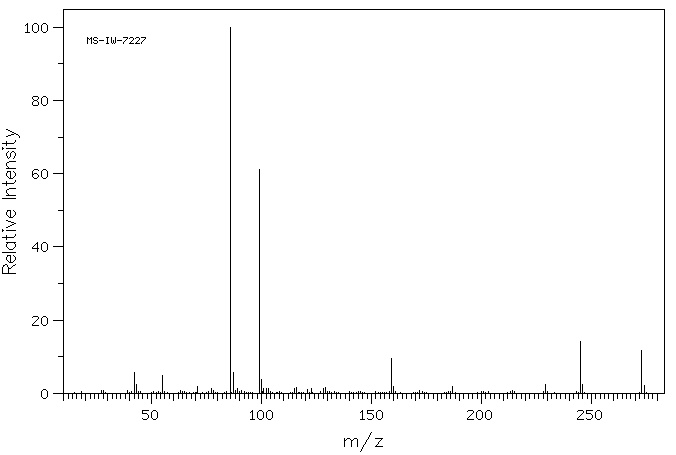
质谱MS
-
碳谱13CNMR
-
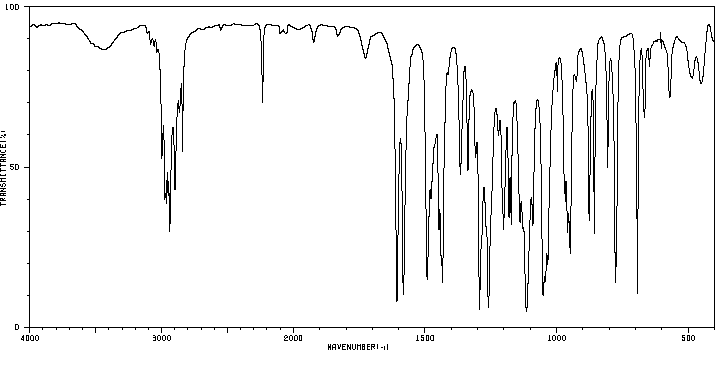
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯