2-(4-甲基苯氧基)乙醇乙酸酯 | 6807-11-0
中文名称
2-(4-甲基苯氧基)乙醇乙酸酯
中文别名
2-对甲苯基乙氧基乙酸盐;二乙酰甲苯;4-(2-乙酰氧基乙氧基)甲苯;乙二醇单对甲苯醚乙酸盐
英文名称
2-(p-tolyloxy)ethyl acetate
英文别名
2-(p-Tolyloxy)-ethylacetat;2-(4-Methylphenoxy)-ethylacetat;p-Kresoxyethylacetat;2-(4-methylphenoxy)ethyl acetate
CAS
6807-11-0
化学式
C11H14O3
mdl
MFCD00059337
分子量
194.23
InChiKey
ZJMLZGKLUMJGSL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:94 °C / 2mmHg
-
密度:1.08
-
LogP:2.191 (est)
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:14
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.363
-
拓扑面积:35.5
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2915390090
SDS
4-(2-Acetoxyethoxy)toluene Revision number: 1
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 4-(2-Acetoxyethoxy)toluene
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
None
Pictograms or hazard symbols
Signal word No signal word
None
Hazard statement
Precautionary statements None
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): 4-(2-Acetoxyethoxy)toluene
Percent: >98.0%(GC)
CAS Number: 6807-11-0
Synonyms: Acetic Acid 2-p-Tolyloxyethyl Ester , Ethylene Glycol Mono-p-tolyl Ether Acetate , 2-
p-Tolyloxyethyl Acetate
Chemical Formula: C11H14O3
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Extinguishing media not to Solid streams of water
be used:
4-(2-Acetoxyethoxy)toluene
Section 5. FIRE-FIGHTING MEASURES
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapor or mist. Wash hands and face thoroughly after handling.
Use a ventilation, local exhaust if vapor or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store away from incompatible materials such as oxidizing agents.
Law is followed.
Packaging material:
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: clear
Color: Colorless - Pale yellow
Odor: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling Point/Range: 94 °C/0.3kPa
Flash Point: No data available
Explosive limits
Lower: No data available
Upper: No data available
Density: 1.08
Solubility: No data available
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
No special reactivity has been reported.
Reactivity:
Incompartible materials: oxidizing agents
4-(2-Acetoxyethoxy)toluene
Section 10. STABILITY AND REACTIVITY
Hazardous Decomposition Carbon monoxide, Carbon dioxide
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobillity in soil
log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not Listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
4-(2-Acetoxyethoxy)toluene
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 4-(2-Acetoxyethoxy)toluene
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
None
Pictograms or hazard symbols
Signal word No signal word
None
Hazard statement
Precautionary statements None
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): 4-(2-Acetoxyethoxy)toluene
Percent: >98.0%(GC)
CAS Number: 6807-11-0
Synonyms: Acetic Acid 2-p-Tolyloxyethyl Ester , Ethylene Glycol Mono-p-tolyl Ether Acetate , 2-
p-Tolyloxyethyl Acetate
Chemical Formula: C11H14O3
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Extinguishing media not to Solid streams of water
be used:
4-(2-Acetoxyethoxy)toluene
Section 5. FIRE-FIGHTING MEASURES
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapor or mist. Wash hands and face thoroughly after handling.
Use a ventilation, local exhaust if vapor or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store away from incompatible materials such as oxidizing agents.
Law is followed.
Packaging material:
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: clear
Color: Colorless - Pale yellow
Odor: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling Point/Range: 94 °C/0.3kPa
Flash Point: No data available
Explosive limits
Lower: No data available
Upper: No data available
Density: 1.08
Solubility: No data available
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
No special reactivity has been reported.
Reactivity:
Incompartible materials: oxidizing agents
4-(2-Acetoxyethoxy)toluene
Section 10. STABILITY AND REACTIVITY
Hazardous Decomposition Carbon monoxide, Carbon dioxide
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobillity in soil
log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not Listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
4-(2-Acetoxyethoxy)toluene
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-(4-甲基苯氧基)乙醇 2-(4-methylphenoxy)ethanol 15149-10-7 C9H12O2 152.193
反应信息
-
作为反应物:描述:参考文献:名称:Synthesis of Dimeric and Trimeric 2-(p-Tolyloxy)ethyl Acetate-Formaldehyde Polymer Model Compounds摘要:合成了二聚和三聚 2-(对聚氧)乙酸乙酯甲醛聚合物模型化合物以及二聚物的卤代化合物。2-(p-tolyloxy)ethyl acetate 与甲醛在酸性介质中反应合成了 2,2′-亚甲基双(5-甲基-1,2-苯氧基)二乙酸乙酯。6-氯-4,4′-二甲基-2,2′-亚甲基二苯酚与 2-氯乙醇反应制备 2-[2-(2-乙酰氧基乙氧基-3-氯-5-甲基苄基)-4-甲基苯氧基]乙酸乙酯、然后用乙酸酐处理,并从 2,6-双(2-羟基-5-甲基苄基)-4-甲基苯酚制备 2-[2,6-双(2-乙酰氧乙氧基-5-甲基苄基)-4-甲基苯氧基]乙酸乙酯。DOI:10.1246/bcsj.47.3102
-
作为产物:描述:2-(4-甲基苯氧基)乙醇 、 溶剂黄146 在 nickel oxinate 作用下, 以 neat (no solvent) 为溶剂, 以96%的产率得到2-(4-甲基苯氧基)乙醇乙酸酯参考文献:名称:镍(II)和铜(II)催化的烷醇胺,苯氧基乙醇和醇的化学选择性O-甲酰基和O-酰基保护摘要:对于具有胺和醇作为反应性官能团的双官能化合物(例如烷醇胺),实现化学选择性始终是至关重要且具有挑战性的。这种反应的例子之一是使烷醇胺的O-甲酰基和O-酰基保护的选择性达到100%。为避免保护和脱保护步骤并克服这一问题,Ni(Ni(Ni))催化了一种新的化学选择性,有效且简单的官能团保护方案,如烷醇胺和苯氧基乙醇的O-甲酰化和O-酰化以及醇和胺之间的竞争性O-选择性。II)和Cu(II)在均相介质中仅以5 mol%的催化剂负载量与8-羟基喹啉形成络合物。在不存在用于室温下以甲酸为甲酰基源的O-甲酰化溶剂和在70°C下以乙酸为酰基源的O-酰化条件下,可获得良好或优异的收率。另外,在该反应过程中产生最少的废水和废物,因为相应的酸的钠盐可以在该过程中回收并可以重复使用。这种化学反应容易耐受各种官能团,如20个对烷醇胺的O-甲酰化和O-酰化具有100%化学选择性的实例以及30个O-甲酰化和在已成功合DOI:10.1039/d0gc00520g
文献信息
-
Nickel catalysis enables convergent paired electrolysis for direct arylation of benzylic C–H bonds作者:Lei Zhang、Xile HuDOI:10.1039/d0sc01445a日期:——intermediates generated at the two opposite electrodes of an electrochemical cell, achieving direct arylation of benzylic C–H bonds. This method yields a diverse set of diarylmethanes, which are important structural motifs in medicinal and materials chemistry. Preliminary mechanistic study suggests oxidation of a benzylic C–H bond, Ni-catalyzed C–C coupling, and reduction of a Ni intermediate as key elements of
-
Sulphonium Salt Initiators申请人:Wolf Jean-Pierre公开号:US20090208872A1公开(公告)日:2009-08-20Compounds of the formula (I), (II), (III), (IV) and wherein, R is hydrogen, C 1 -C 20 alkyl; C 2 -C 20 alkyl interrupted by one or more O; is -L-X—R 2 or -L-R 2 ; R 1 has for example one of the meanings as given for R; R 2 is a monovalent sensitizer or photoinitiator moiety; Ar 1 and Ar 2 for example independently of one another are phenyl substituted by C 1 -C 20 alkyl, halogen or OR 3 ; or are unsubstituted naphthyl, anthryl, phenanthryl or biphenylyl; or are naphthyl, anthryl, phenanthryl or biphenylyl substituted by C 1 -C 20 alkyl, OH or OR 3 ; or are —Ar 4 -A-Ar 3 ; Ar 3 is unsubstituted phenyl naphthyl, anthryl, phenanthryl or biphenylyl; or is phenyl, naphthyl, anthryl, phenanthryl or biphenylyl substituted by C 1 -C 20 alkyl, OR 3 or benzoyl; Ar 4 is phenylene, naphthylene, anthrylene or phenanthrylene; A is a direct bond, S, O or C 1 -C 20 alkylene; X is CO, C(O)O, OC(O), O, S or NR 3 ; L is C 1 -C 20 alkylene or C 2 -C 20 alkylene interrupted by one or more O; R 3 is C 1 -C 20 alkyl or C 1 -C 20 hydroxyalkyl; and Y is an anion, are suitable as photolatent acid generators.公式(I),(II),(III),(IV)的化合物,其中R是氢,C1-C20烷基; C2-C20烷基中断一个或多个O;为-L-X—R2或-L-R2; R1例如具有给定为R的含义之一; R2是单价敏化剂或光引发剂基团; Ar1和Ar2例如独立地是被C1-C20烷基,卤素或OR3取代的苯基;或未取代的萘基,蒽基,菲基或联苯基;或被C1-C20烷基,OH或OR3取代的萘基,蒽基,菲基或联苯基;或是-Ar4-A-Ar3; Ar3是未取代的苯基,萘基,蒽基,菲基或联苯基;或是被C1-C20烷基,OR3或苯甲酰取代的苯基,萘基,蒽基,菲基或联苯基; Ar4是苯撑,萘撑,蒽撑或菲撑; A是直接键,S,O或C1-C20烷基; X是CO,C(O)O,OC(O),O,S或NR3; L是C1-C20烷基或C2-C20烷基中断一个或多个O; R3是C1-C20烷基或C1-C20羟基烷基; Y是阴离子,适用作光潜酸发生剂。
-
Bromine radical-enhanced HAT activity leading to stoichiometric couplings of methylarenes with acid chlorides作者:Qiao-Lin Wang、Huawen Huang、Guojiang Mao、Guo-Jun DengDOI:10.1039/d2gc02972c日期:——The stoichiometric coupling of readily available methylarenes and diverse acid chlorides into α-aryl ketones, a valued structural motif present in pharmacologically relevant molecules, has been developed. The utility of this protocol is demonstrated by the late-stage acylation of biologically active molecules in a stoichiometric manner. Mechanistic investigation reveals that the photocatalytically
-
INK COMPOSITION FOR ORGANIC LIGHT EMITTING DEVICE申请人:LG CHEM, LTD.公开号:EP3789464A1公开(公告)日:2021-03-10The present invention relates to an ink composition for an organic light emitting device that can be applied to an inkjet process. When this is applied to an inkjet process, it can form a flat film with a smooth surface when dried after forming the ink film.本发明涉及一种可用于喷墨工艺的有机发光器件墨水组合物。将其应用于喷墨工艺时,可在形成墨膜后干燥时形成表面光滑的平膜。
-
Catalytic Hydroxyethylation of Phenols with Renewable Ethylene Glycol Diester as an Alternative to Ethylene Oxide作者:Yu‐Ji Luo、Zhi LiDOI:10.1002/ejoc.202400158日期:2024.6.24A Lewis acid-catalyzed, solvent-free hydroxyethylation strategy that converts phenols into phenoxyethanol esters is reported. Ethylene glycol diesters are used as renewable and safe alternatives to the harmful ethylene oxide. Oligomerization byproducts from ethylene oxide are also avoided. Applications are demonstrated in upcycling of polyester plastics and preparation of bioactive chemicals.
表征谱图
-
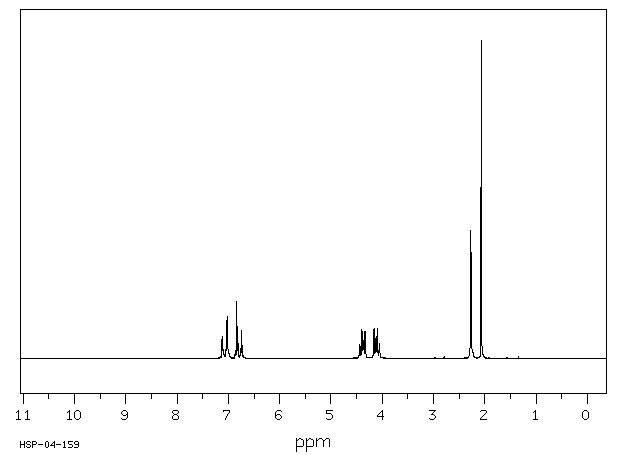
氢谱1HNMR
-
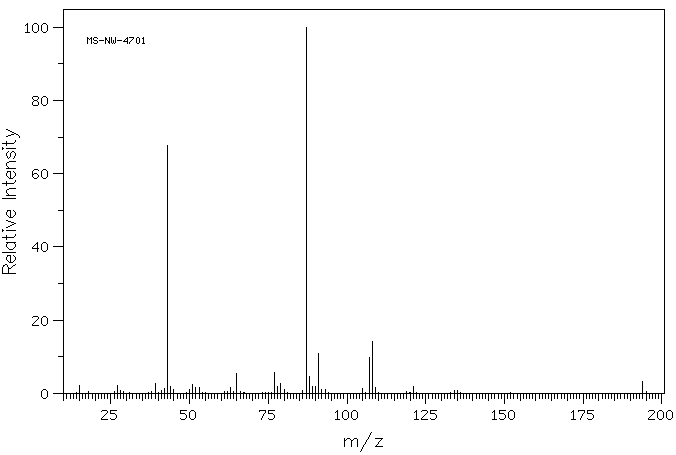
质谱MS
-
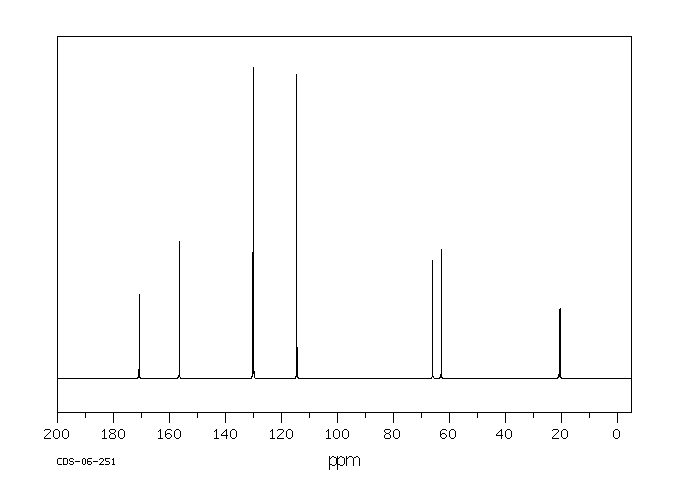
碳谱13CNMR
-
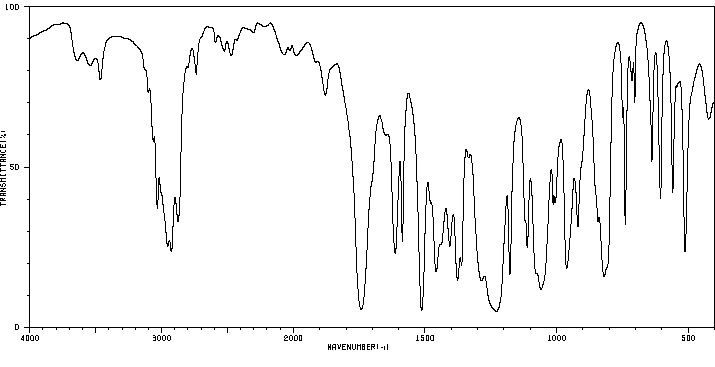
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯