3-(2-phenethyl)cyclohexan-1-one | 108011-20-7
中文名称
——
中文别名
——
英文名称
3-(2-phenethyl)cyclohexan-1-one
英文别名
3-(2-phenethyl)cyclohexanone;3-phenethylcyclohexanone;3-phenethyl-cyclohexanone;3-Phenaethyl-cyclohexanon;Cyclohexanone, 3-(2-phenylethyl)-;3-(2-phenylethyl)cyclohexan-1-one
CAS
108011-20-7
化学式
C14H18O
mdl
——
分子量
202.296
InChiKey
BENRBZIPFOWPND-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:155 °C(Press: 5 Torr)
-
密度:1.005±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:15
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
SDS
反应信息
-
作为反应物:描述:3-(2-phenethyl)cyclohexan-1-one 在 ruthenium trichloride 、 sodium periodate 作用下, 以 四氯化碳 、 水 、 乙腈 为溶剂, 反应 19.0h, 以63%的产率得到(+/-)-3-(3-oxocyclohexyl)propionic acid参考文献:名称:格氏试剂对环状α,β-不饱和羰基化合物的催化对映选择性共轭加成摘要:由铜盐,手性膦和格氏试剂与环己烯酮产生的有机铜试剂的催化不对称共轭加成反应高度依赖于铜离子,溶剂,格氏试剂和手性膦结构的抗衡阴离子。在-78°C下,使用8摩尔%的碘化铜,32摩尔%的氨基膦3和1.2当量的有机氯化镁与环己烯酮在乙醚中的混合物进行反应,得到3-取代的环己酮,其ee最高为92%,ee为90 % 屈服。DOI:10.1016/s0040-4020(99)00095-2
-
作为产物:描述:3-乙氧基-2-环己烯-1-酮 在 1,3-bis(2,6-diisopropylphenyl)imidazolium copper(I) chloride sodium t-butanolate 、 poly(methylhydrosiloxane) 作用下, 以 四氢呋喃 、 甲苯 为溶剂, 反应 17.0h, 生成 3-(2-phenethyl)cyclohexan-1-one参考文献:名称:铜卡宾络合物催化的α,β-不饱和羰基化合物的共轭还原。摘要:[反应:见正文]制备了N-杂环卡宾氯化铜(NHC-CuCl)配合物(2),并用于催化α,β-不饱和羰基化合物的共轭还原。催化量的2和NaOt-Bu与作为化学计量还原剂的聚(甲基氢硅氧烷)(PMHS)的结合产生了一种活性催化剂,用于1,4-还原三和四取代的α,β-不饱和酯和环状烯酮。活性催化物质也可以在NaOt-Bu和PMHS存在下由1,3-双(2,6-二异丙基苯基)-咪唑鎓氯化物(1)CuCl(2).2H(2)O原位生成。DOI:10.1021/ol034560p
文献信息
-
Highly Efficient Synthesis of Terminal Alkenes from Ketones作者:Hélène Lebel、Danielle Guay、Valérie Paquet、Kim HuardDOI:10.1021/ol049085p日期:2004.9.1[reaction: see text] The rhodium(I)-catalyzed methylenation of ketones using trimethylsilyldiazomethane proceeds to give the corresponding alkenes in good yields (60-97%). The use of an excess of 2-propanol and 1,4-dioxane as a solvent were instrumental to obtain the desired alkenes in high yields. Superior results were achieved with the rhodium(I)-catalyzed methylenation in comparison with the standard
-
Rhodium-Catalyzed Synthesis of Terminal Alkenes作者:Hélène Lebel、Valérie PaquetDOI:10.1055/s-2004-834920日期:——Terminal alkenes have been efficiently prepared via a rhodium-catalyzed olefination procedure using Wilkinson’s catalyst in the presence of triphenylphosphine, 2-propanol and trimethylsilyldiazomethane. Optimized reaction conditions are described for aldehydes and ketones, as well as alternative work up procedures.
-
Transmetalation reactions of organosamarium reagents. Chlorosilane-accelerated copper-catalyzed conjugate additions作者:Peter Wipf、Srikanth VenkatramanDOI:10.1021/jo00064a040日期:1993.6TMSCl accelerates the conjugate addition of in situ prepared organosamarium reagents to alpha,beta-unsaturated carbonyl compounds and nitriles in the presence of HMPA and catalytic amounts of Cu(I) salts. Reactions at -78-degrees-C leads to silyl enol ethers which are isolated or cleaved with TBAF to give beta-alkylated ketones in 30-90% overall yield. Catalysis is most efficient in the presence of 4 equiv of TMSCl and HMPA. HMPA is also necessary for the in situ preparation of the organosamarium species from alkyl halide and SmI2. Some functional groups (chloride,ether,alkene, amide) are tolerated in this process. In the absence of Cu(I) salts, 1,2-additions of organosamarium reagents to carbonyl groups are also dramatically accelerated by TMSCl/HMPA and occur within minutes at -78-degrees-C.
-
Conjugate addition reactions of organosamarium species via in situ transmetalation to copper(I) salts作者:Michael J. Totleben、Dennis P. Curran、Peter WipfDOI:10.1021/jo00032a027日期:1992.3Preformed organosamarium species, available by reduction of aryl or alkyl halides with SmI2, were treated with copper(I) salts to effect in situ transmetalation and conjugate addition to enones. In a series of copper(I) salts, CuI.P(OEt)3 gave best results in combination with 2 equiv of organosamarium reagent. This new method allows the multiple formation of carbon-carbon bonds through a combination of radical and cuprate chemistry.
-
Dehydration of β-Phenylethylcyclohexanol-3作者:B. C. PalDOI:10.1021/ja01617a079日期:1955.6
表征谱图
-
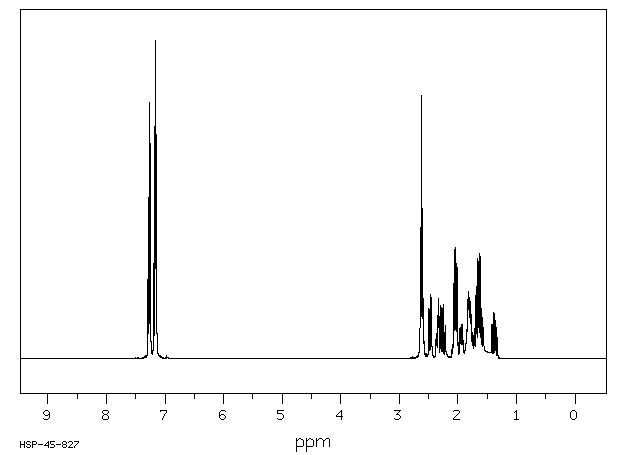
氢谱1HNMR
-
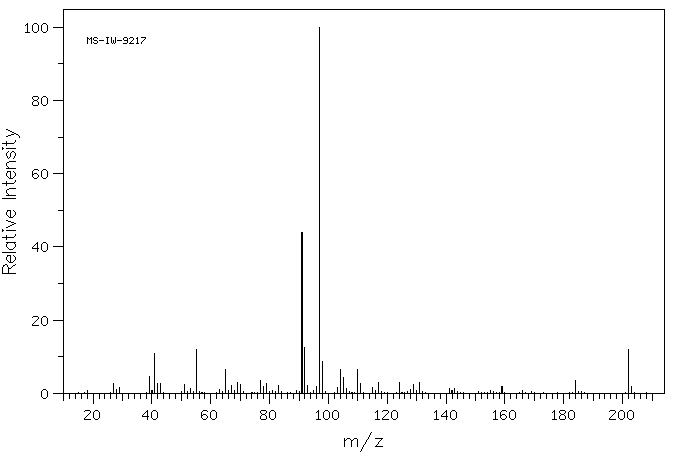
质谱MS
-
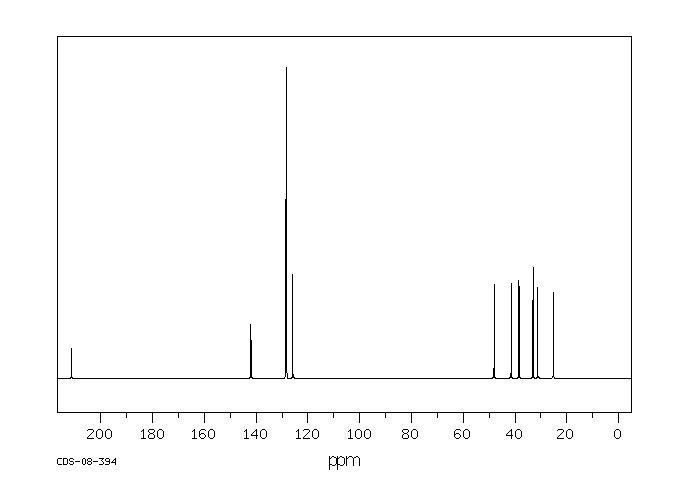
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫