2,5-二(4-甲氧基苯基)-2,5-环己二烯-1,4-二酮 | 5333-03-9
中文名称
2,5-二(4-甲氧基苯基)-2,5-环己二烯-1,4-二酮
中文别名
——
英文名称
2,5-bis-(4-methoxyphenyl)-1,4-benzoquinone
英文别名
2,5-bis-(4-methoxy-phenyl)-[1,4]benzoquinone;2,5-Bis-(4-methoxy-phenyl)-[1,4]benzochinon;2,5-bis(4-methoxyphenyl)-2,5-cyclohexadiene-1,4-dione;2,5-Cyclohexadiene-1,4-dione, 2,5-bis(4-methoxyphenyl)-;2,5-bis(4-methoxyphenyl)cyclohexa-2,5-diene-1,4-dione
CAS
5333-03-9
化学式
C20H16O4
mdl
——
分子量
320.345
InChiKey
JZJYUHYPMJANNP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:24
-
可旋转键数:4
-
环数:3.0
-
sp3杂化的碳原子比例:0.1
-
拓扑面积:52.6
-
氢给体数:0
-
氢受体数:4
安全信息
-
海关编码:2914509090
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:曲霉的有毒代谢产物的结构。I.化合物A和E,细胞毒性对三联苯。摘要:通过光谱数据、氧化反应和化合物 A 三甲基醚的合成,阐明了特征性细胞毒性化合物 A(三苯乙烯)和 E(脱氧三苯乙烯)的结构,即对三苯乙烯衍生物(1 和 24)。此外,还讨论了这些化合物及其衍生物的细胞毒性。DOI:10.1248/cpb.24.613
-
作为产物:描述:参考文献:名称:Akagi; Hirose, Yakugaku Zasshi/Journal of the Pharmaceutical Society of Japan, 1942, vol. 62, p. 191,193摘要:DOI:
文献信息
-
Studies on the Meerwein Arylation-Based Preparation of 2,3-Diarylbenzene-1,4-diones and Its Theoretical Interpretation作者:Ichiro Takahashi、Osamu Muramatsu、Jun Fukuhara、Yoshinao Hosokawa、Naohiko Takeyama、Toshio Morita、Hidehiko KitajimaDOI:10.1246/cl.1994.465日期:1994.3Title compounds possessing a variety of substituents are systematically prepared by means of Meerwein arylation of benzene-1,4-dione. Synthetic aspects and their MNDO calculation-based interpretation are described.通过苯-1,4-二酮的米尔韦恩芳构化,可以系统地制备具有各种取代基的标题化合物。本文介绍了合成方法及其基于MNDO计算的解释。
-
Planar Chiral Rhodium Complexes of 1,4‐Benzoquinones作者:Nikita M. Ankudinov、Yulia V. Nelyubina、Dmitry S. PerekalinDOI:10.1002/chem.202200195日期:2022.3.28Chiral rhodium complexes are widely used in asymmetric catalysis. Herein we describe a new approach to such chiral complexes featuring the intrinsically achiral benzoquinone ligands. The synthesis was achieved by the face-selective coordination of 2,5-substituted benzoquinones with rhodium bearing the readily available oxazoline-phenolate ligand. This chiral auxiliary ligand was subsequently replaced
-
Efficient Synthesis of Symmetrical 2,5-Disubstituted Benzoquinones via Palladium-Catalyzed Double Negishi Coupling作者:Andreas Palmgren、Atli Thorarensen、Jan-E. BäckvallDOI:10.1021/jo9721812日期:1998.5.1
-
Synthesis and evaluation of arylquinones as BACE1 inhibitors, β-amyloid peptide aggregation inhibitors, and destabilizers of preformed β-amyloid fibrils作者:Andrea Ortega、Ángela Rincón、Karim L. Jiménez-Aliaga、Paloma Bermejo-Bescós、Sagrario Martín-Aragón、María Teresa Molina、Aurelio G. CsákÿDOI:10.1016/j.bmcl.2011.03.023日期:2011.4BACE1 activity, inhibition of A beta aggregation, and disaggregation of preformed A beta fibrils constitute the three major targets in the development of small-molecule lipophilic new drugs for the treatment of Alzheimer's disease (AD). Quinones are widely distributed among natural products and possess relevant and varied biological activities including antitumor and antibiotic, inhibition of HIV-1 reverse transcriptase, antidiabetic, or COX-inhibition, among others. We report herein the interaction of several arylquinones and their derivatives with the amyloidogenic pathway of the amyloid precursor protein processing. Our studies put forward that these compounds are promising candidates in the development of new drugs which are effective simultaneously towards the three major targets of AD. (C) 2011 Elsevier Ltd. All rights reserved.
-
DE459739申请人:——公开号:——公开(公告)日:——
表征谱图
-
氢谱1HNMR
-
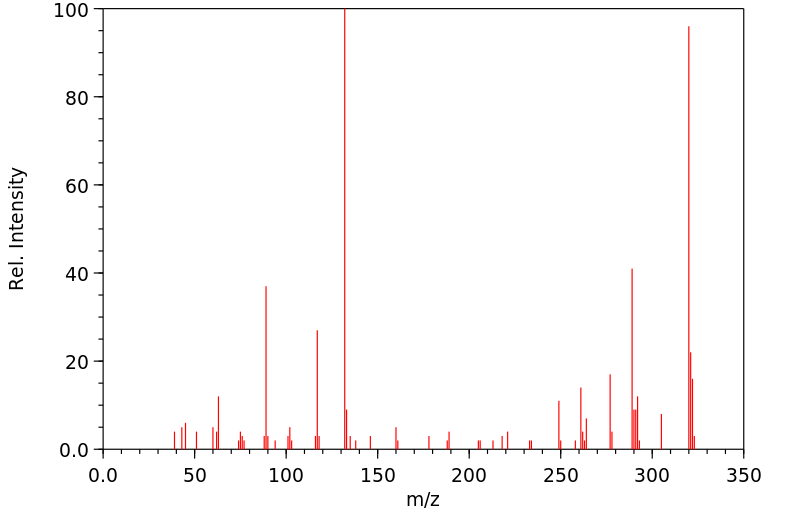
质谱MS
-
碳谱13CNMR
-
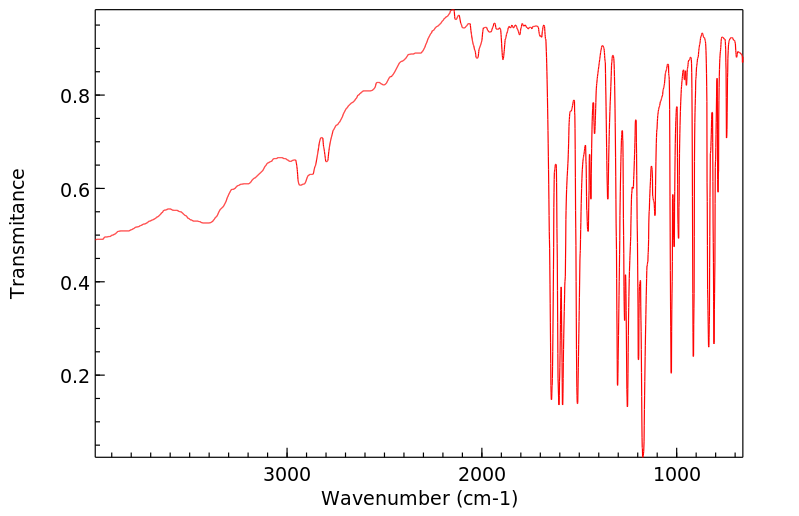
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯