(+/-)-2-Ethylamino-3-phenyl-propan-1-ol | 24629-32-1
中文名称
——
中文别名
——
英文名称
(+/-)-2-Ethylamino-3-phenyl-propan-1-ol
英文别名
N-Aethyl-DL-phenyl-alaninol;N-Ethyl-DL-phenylalaninol;2-(Ethylamino)-3-phenylpropan-1-ol
CAS
24629-32-1
化学式
C11H17NO
mdl
——
分子量
179.262
InChiKey
VMJALMGSTUCUFE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:13
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.45
-
拓扑面积:32.3
-
氢给体数:2
-
氢受体数:2
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-乙酰-DL-苯丙氨酸 (R,S)-N-acetyl phenylalanine 2901-75-9 C11H13NO3 207.229
反应信息
-
作为反应物:描述:参考文献:名称:Yonemitsu,O. et al., Chemical and pharmaceutical bulletin, 1969, vol. 17, p. 2075 - 2082摘要:DOI:
-
作为产物:描述:1-氯-4-苯基-3-氮杂-3-丁烯 在 2,6-二甲基吡啶 、 lithium aluminium tetrahydride 作用下, 以 四氢呋喃 、 苯 为溶剂, 反应 15.0h, 生成 (+/-)-2-Ethylamino-3-phenyl-propan-1-ol参考文献:名称:Transformation of trans-4-Aryl-3-chloro-1-(2-chloroethyl)azetidin-2-ones into 3-Aryl-2-(ethylamino)propan-1-ols via Intermediate 1-(1-Aryl-2-chloro-3-hydroxypropyl)aziridines and trans-2-Aryl-3-(hydroxymethyl)aziridines摘要:trans-4-Aryl-3-chloro-1-(2-chloroethyl)azetidin-2-ones, prepared through cyclocondensation of chloroketene and the appropriate imines in a diastereoselective way, were unexpectedly transformed into 3-aryl-2-(ethylamino)propan-1-ols using LiAlH4 in THF under reflux. A stepwise analysis showed that the initially formed 1-(1-aryl-2-chloro-3-hydroxypropyl)aziridines were converted into trans-2-aryl-3-(hydroxymethyl)aziridines, most probably via N-spiro bis-aziridinium intermediates, which were subsequently prone to undergo ring opening by LiAlH4 to afford 3-aryl-2-(ethylamino)propan-1-ols.DOI:10.1021/jo1020932
文献信息
-
Cannabinoid receptor ligands and uses thereof申请人:Pfizer Inc.公开号:US20040214837A1公开(公告)日:2004-10-28Compounds of Formula (I) that act as cannabinoid receptor ligands and their uses in the treatment of diseases linked to the mediation of the cannabinoid receptors in animals are described herein. 1
-
Amino acids and peptides. IV. Selective reduction of peptide-ester groups作者:Osamu Yonemitsu、Tatsuo Hamada、Yuichi KanaokaDOI:10.1016/s0040-4039(00)75503-1日期:1968.1
-
1,2,3,4-Tetrahydroisoquinoline-pyrrolidine derivatives as antagonists of apoptosis (IAPs) for the treatment of cancer.申请人:Bristol-Myers Squibb Company公开号:EP2861581B1公开(公告)日:2017-02-01
-
US7268133B2申请人:——公开号:US7268133B2公开(公告)日:2007-09-11
表征谱图
-
氢谱1HNMR
-
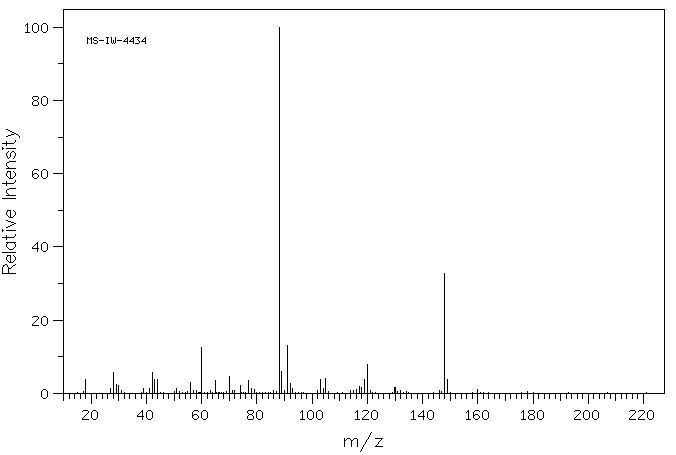
质谱MS
-
碳谱13CNMR
-
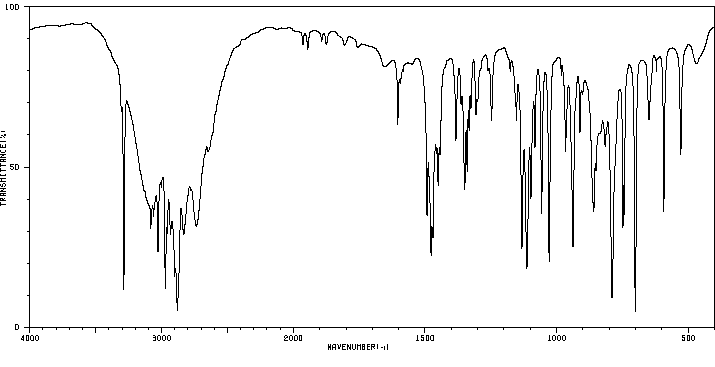
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫