(E)-tagetone | 6752-80-3
中文名称
——
中文别名
——
英文名称
(E)-tagetone
英文别名
trans-tagetone;tagetone;(E)-2,6-Dimethylocta-5,7-dien-4-one;(5E)-2,6-dimethylocta-5,7-dien-4-one
CAS
6752-80-3
化学式
C10H16O
mdl
——
分子量
152.236
InChiKey
RJXKHBTYHGBOKV-VQHVLOKHSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:222.9±9.0 °C(Predicted)
-
密度:0.847±0.06 g/cm3(Predicted)
-
LogP:2.543 (est)
-
保留指数:1136;1120;1132;1129;1122;1135;1125
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:11
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
反应信息
-
作为产物:描述:参考文献:名称:Vig; Bari; Sattar, Journal of the Indian Chemical Society, 1989, vol. 66, # 2, p. 98 - 100摘要:DOI:
文献信息
-
Stereoselective synthesis of (E)-α-hydroxy-1,3-dienes via the reaction of 2,5-dihydrothiophene S,S-dioxides with carbonyl compounds作者:Sachiko Yamada、Hiromasa Suzuki、Hiroyuki Naito、Takashi Nomoto、Hiroaki TakayamaDOI:10.1039/c39870000332日期:——A stereoselective synthesis of (E)-α-hydroxy-1,3-dienes, by thermal extrusion from adducts of 2,5-dihydrothiophene S,S-dioxide (1) with aldehydes and ketones, is presented and its extension to the synthesis of the dienone, (E)-tagetone, and of a 1,3,5-triene system, is illustrated.
-
Trityl Salt Catalyzed Aldol Reaction between α,β-Acetylenic Ketones and Silyl Enol Ethers作者:Shu Kobayashi、Shigekazu Matsui、Teruaki MukaiyamaDOI:10.1246/cl.1988.1491日期:1988.9.5In the presence of a catalytic amount of trityl salt, α,β-acetylenic ketones react with silyl enol ethers to afford the corresponding aldol adducts (1,2-addition products) stereoselectively in high yields. Naturally occurring monoterpenes, trans- and cis-tagetones, are synthesized by the use of this reaction.在催化量的三苯甲基盐存在下,α,β-炔酮与甲硅烷基烯醇醚反应以高产率立体选择性地提供相应的羟醛加合物(1,2-加成产物)。天然存在的单萜、反式和顺式-万寿菊内酯是通过使用该反应合成的。
-
Perfumery compounds and their preparation申请人:BUSH BOAKE ALLEN Limited公开号:EP0141569A2公开(公告)日:1985-05-15Useful perfumery compounds have the formula where there is a double bond in one of the 5,6 or 6,7 or 7,8 positions. The compounds may be made by reaction of 2-methyl-pentan-4-one with isobutyraldehyde followed by dehydration, preferably in the presence of a weak acid.
-
Isager, Per; Thomsen, Ib; Torssell, Kurt B. G., Acta Chemica Scandinavica, 1990, vol. 44, p. 806 - 813作者:Isager, Per、Thomsen, Ib、Torssell, Kurt B. G.DOI:——日期:——
-
Melikyan, G. G.; Babayan, E. V.; Atanesyan, K. A., Journal of Organic Chemistry USSR (English Translation), 1984, p. 1884 - 1889作者:Melikyan, G. G.、Babayan, E. V.、Atanesyan, K. A.、Badanyan, Sh. O.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
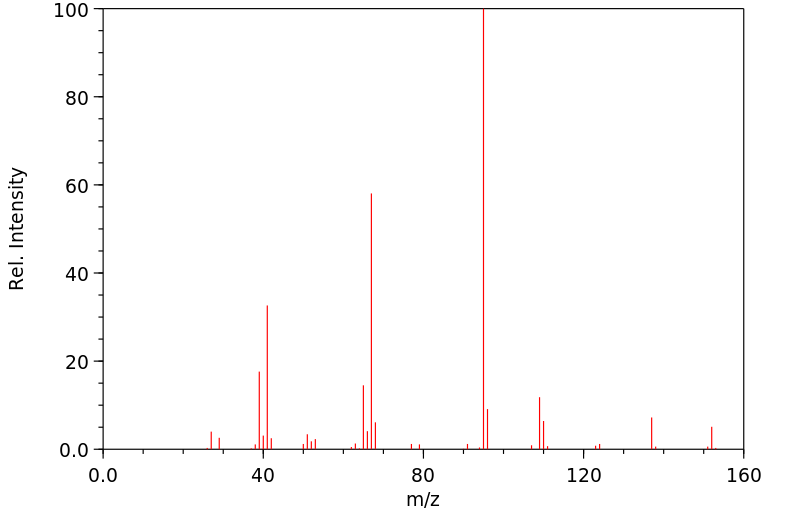
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷