3-乙酰氨基香豆素 | 779-30-6
中文名称
3-乙酰氨基香豆素
中文别名
——
英文名称
3-acetamidocoumarin
英文别名
N-(2-oxo-2H-chromen-3-yl)acetamide;3-Acetamino-cumarin;3-N-acetylaminocoumarin;3-acetylaminocoumarin;N-(2-oxochromen-3-yl)acetamide
CAS
779-30-6
化学式
C11H9NO3
mdl
MFCD00075556
分子量
203.197
InChiKey
XJYLCCJIDYSFMB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:205-207 °C(lit.)
-
沸点:482.0±45.0 °C(Predicted)
-
密度:1.31±0.1 g/cm3(Predicted)
-
稳定性/保质期:
在常温常压下稳定。
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:15
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.09
-
拓扑面积:55.4
-
氢给体数:1
-
氢受体数:3
安全信息
-
WGK Germany:3
-
安全说明:S22,S24/25
-
海关编码:2932209090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
SDS
| Name: | 3-Acetamidocoumarin Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 779-30-6 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 779-30-6 | 3-Acetamidocoumarin | >98 % | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
The toxicological properties of this substance have not been fully investigated.
Inhalation:
The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up, then place into a suitable container for disposal.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Keep container closed when not in use.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate general or local exhaust ventilation to keep airborne concentrations below the permissible exposure limits.
Exposure Limits CAS# 779-30-6: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves and clothing to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: Not available.
Odor: none reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 206 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C11H9NO3
Molecular Weight: 203.20
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidants.
Hazardous Decomposition Products:
Irritating and toxic fumes and gases.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 779-30-6 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3-Acetamidocoumarin - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 779-30-6: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 779-30-6 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 779-30-6 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-氨基香豆素 aminocoumarine 1635-31-0 C9H7NO2 161.16 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-bromo-N-(2-oxo-2H-chromen-3-yl)acetamide 1365534-05-9 C11H8BrNO3 282.093 —— 3-trifluoracetamido-2H-chromen-2-one —— C11H6F3NO3 257.169 —— N-(2-oxo-2H-chromen-3-yl)-3-phenylacrylamide 76461-89-7 C18H13NO3 291.306 —— 3-(4-chlorophenyl)-N-(2-oxo-2H-chromen-3-yl)acrylamide 330662-51-6 C18H12ClNO3 325.751 —— (2-oxo-2H-chromen-3-yl)carbamic acid tert-butyl ester 205239-07-2 C14H15NO4 261.277 3-氨基香豆素 aminocoumarine 1635-31-0 C9H7NO2 161.16 —— 3-azidocoumarin 152711-55-2 C9H5N3O2 187.158 2H-1-苯并吡喃-2-酮,6-氨基- 6-aminocoumarin 14415-44-2 C9H7NO2 161.16 N-亚水杨基-3-氨基香豆素 3-[(2-hydroxy-benzylidene)amino]chromen-2-one 1473-60-5 C16H11NO3 265.268
反应信息
-
作为反应物:描述:3-乙酰氨基香豆素 在 盐酸 、 溶剂黄146 、 sodium nitrite 作用下, 以 乙醇 为溶剂, 反应 8.0h, 生成 4-(dimethylamino)-N'-(2-oxochromen-3-yl)-N-phenyliminobenzenecarboximidamide参考文献:名称:Kumar, Atul; Verma, M.; Saxena, A. K., Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 1987, vol. 26, # 1-12, p. 378 - 380摘要:DOI:
-
作为产物:描述:alkaline earth salt of/the/ methylsulfuric acid 在 sodium hydroxide 作用下, 生成 3-乙酰氨基香豆素参考文献:名称:Dakin, Journal of Biological Chemistry, 1929, vol. 82, p. 445摘要:DOI:
文献信息
-
Evaluation of novel coumarin-proline sulfonamide hybrids as anticancer and antidiabetic agents作者:Sunil Dutt Durgapal、Shubhangi S. SomanDOI:10.1080/00397911.2019.1647439日期:——available for treatment of cancer and diabetes individually, peptide linkage containing proline sulfonamide can be a promising therapy for treatment of both cancer as well as diabetes. Here, we report design and synthesis of novel coumarin-proline sulfonamide derivatives as anticancer and antidiabetic agents. All the synthesized compounds were screened for their anticancer activity against lungs cancer摘要 癌症和糖尿病被认为是世界范围内影响人类健康的两大疾病。多种疗法可分别用于治疗癌症和糖尿病,含有脯氨酸磺酰胺的肽键可以成为治疗癌症和糖尿病的有前途的疗法。在这里,我们报告了作为抗癌和抗糖尿病药物的新型香豆素-脯氨酸磺酰胺衍生物的设计和合成。使用 3-(4,5-二甲基噻唑-2-基)-2,5-二苯基四唑鎓溴化物染料 (MTT) 筛选所有合成化合物对肺癌细胞系 (A549) 和乳腺癌细胞系 (MCF7) 的抗癌活性) 测定和使用 DPP-IV 抑制测定的抗糖尿病活性。化合物 16b 对乳腺癌细胞系 (MCF7) 显示出优异的活性,IC50 值为 1.07 µM。所有化合物均表现出中等的 DPP-IV 抑制作用。图形概要
-
Benzyne Click Chemistry: Synthesis of Benzotriazoles from Benzynes and Azides作者:Feng Shi、Jesse P. Waldo、Yu Chen、Richard C. LarockDOI:10.1021/ol800675u日期:2008.6.1A variety of substituted benzotriazoles have been prepared by the [3 + 2] cycloaddition of azides to benzynes. The reaction scope is quite general, affording a rapid and easy entry to substituted, functionalized benzotriazoles under mild conditions.多种取代的苯并三唑已通过叠氮化物与苄的 [3 + 2] 环加成反应制备。反应范围非常广泛,可以在温和的条件下快速轻松地获得取代的、官能化的苯并三唑。
-
Copper(<scp>i</scp>)-promoted cycloalkylation–peroxidation of unactivated alkenes via sp<sup>3</sup>C–H functionalisation作者:Arghya Banerjee、Sourav Kumar Santra、Aniket Mishra、Nilufa Khatun、Bhisma K. PatelDOI:10.1039/c4ob01962h日期:——been developed via a three-component reaction involving cycloalkanes, tert-butyl hydroperoxide (TBHP) and internal conjugated alkenes possessing electron-withdrawing groups (EWGs). This process installs C–O and C–C bonds via sp3 C–H functionalisation with concomitant generation of two stereocentres. This regioselective radical addition of coumarin system is opposite to that of styrene.
-
Design and synthesis of aminocoumarin derivatives as DPP-IV inhibitors and anticancer agents作者:Rina Soni、Shubhangi S. SomanDOI:10.1016/j.bioorg.2018.05.008日期:2018.9role in earlier stages of cancer. Here we have reported design, synthesis and applications of aminocoumarin derivatives as DPP-IV inhibitors. Compounds have been synthesized and studied for their DPP-IV inhibition activity. Three compounds have shown moderate inhibition at 100 µM concentration. All compounds were also screened for their anticancer activity against A549 (Lung cancer cell line), MCF-7 (Breast
-
Enantioselective allene/enone photocycloadditions: The use of an inexpensive optically active 1,3-disubstituted allene作者:Mary S. Shepard、Erick M. CarreiraDOI:10.1016/s0040-4020(97)01013-2日期:1997.12and use of an inexpensive readily prepared optically active 1,3-disubstituted allene that may be utilized for enantioselective intramolecular allene/enone photocycloadditions. In addition, we describe novel substrates for intramolecular [2+2] photocycloadditions which substantially expand the scope of the process to include amino- and thio- tethered allene/enones.
表征谱图
-
氢谱1HNMR
-
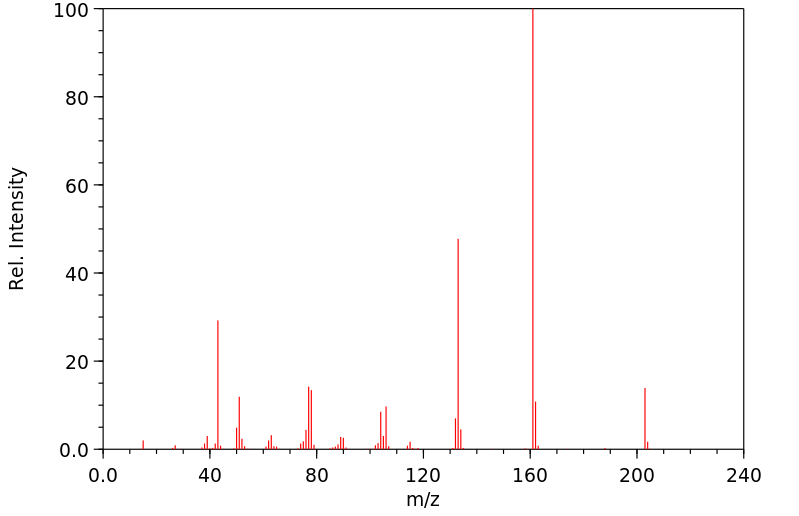
质谱MS
-
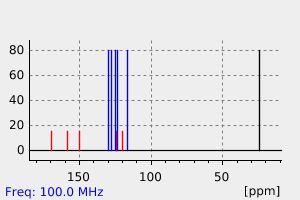
碳谱13CNMR
-
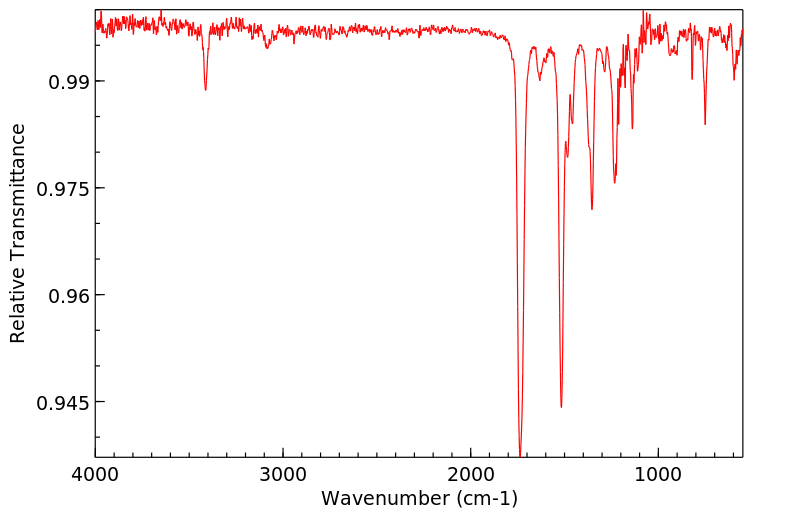
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄皮香豆精
黄木亭
黄曲霉素P2
黄曲霉素P1
黄曲霉素G2-13C17-同位素
黄曲霉素G2
黄曲霉素G1-13C17-同位素
黄曲霉素B2-13C17-同位素
黄曲霉素B1-13C17-同位素
黄曲霉素B1 8,9-环氧化物
黄曲霉素 G1
黄曲霉毒醇Ⅱ
黄曲霉毒醇M1
黄曲霉毒醇A
黄曲霉毒素M2
黄曲霉毒素M1-(O-羧甲基)肟
黄曲霉毒素G2a
黄曲霉毒素G19,10-环氧化物
黄曲霉毒素B2
黄曲霉毒素B1二氯化物
黄曲霉毒素B1-8,9-二氯化物
黄曲霉毒素B1-(O-羧甲基)肟
黄曲霉毒素 Q1
黄曲霉毒素 M1
黄曲霉毒素 B2
黄曲霉毒素 B1
黄曲霉毒素
香豆霉素
香豆素6H
香豆素545T
香豆素545
香豆素525
香豆素343甲酯
香豆素338
香豆素314T
香豆素175
香豆素152
香豆素106
香豆素-D4
香豆素-6-磺酰氯
香豆素-6-甲醛
香豆素-5-氧丁酸
香豆素-4-乙酸
香豆素-3腈
香豆素-35
香豆素-3-羧酸酸酐
香豆素-3-羧酸琥珀酰亚胺酯
香豆素-3-羧酸乙酯
香豆素-3-羧酸
香豆素-3-甲酰氯