环戊基丙二酸二甲酯 | 82491-60-9
中文名称
环戊基丙二酸二甲酯
中文别名
——
英文名称
dimethyl 2-cyclopentylmalonate
英文别名
Dimethyl Cyclopentylmalonate;dimethyl 2-cyclopentylpropanedioate
CAS
82491-60-9
化学式
C10H16O4
mdl
MFCD03844781
分子量
200.235
InChiKey
KCCNEJJMJHDVCN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
密度:1,09 g/cm3
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:14
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:52.6
-
氢给体数:0
-
氢受体数:4
安全信息
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
海关编码:2917209090
-
危险性防范说明:P264,P270,P301+P312,P330
-
危险性描述:H302
SDS
Dimethyl Cyclopentylmalonate Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Dimethyl Cyclopentylmalonate
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Dimethyl Cyclopentylmalonate
Percent: >97.0%(GC)
CAS Number: 82491-60-9
Synonyms: Cyclopentylmalonic Acid Dimethyl Ester
Chemical Formula: C10H16O4
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Unsuitable extinguishing Solid streams of water
media:
Dimethyl Cyclopentylmalonate
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a ventilation, local exhaust if vapour or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Colorless - Almost colorless
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
No data available
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
1.09
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
Dimethyl Cyclopentylmalonate
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
No data available
Log Pow:
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Does not correspond to the classification standard of the United Nations
Hazards Class:
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
Dimethyl Cyclopentylmalonate
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Dimethyl Cyclopentylmalonate
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Dimethyl Cyclopentylmalonate
Percent: >97.0%(GC)
CAS Number: 82491-60-9
Synonyms: Cyclopentylmalonic Acid Dimethyl Ester
Chemical Formula: C10H16O4
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Unsuitable extinguishing Solid streams of water
media:
Dimethyl Cyclopentylmalonate
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a ventilation, local exhaust if vapour or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Colorless - Almost colorless
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
No data available
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
1.09
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
Dimethyl Cyclopentylmalonate
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
No data available
Log Pow:
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Does not correspond to the classification standard of the United Nations
Hazards Class:
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
Dimethyl Cyclopentylmalonate
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:参考文献:名称:解读环戊烷和环己烷衍生物脂肪族 C-H 键氧化的反应性和选择性模式摘要:已经进行了枯草氧基自由基与单取代环戊烷和环己烷反应的动力学、产物和计算研究。HAT 速率、C-H 键氧化的位点选择性和 DFT 计算提供了定量信息和理论模型来解释观察到的模式。环戊烷主要在 C-1 处官能化,从甲基和叔丁基环戊烷到苯基环戊烷,叔 C-H 键活化能垒降低,与计算的 C-H BDE 一致。对于环己烷,从 C-1 开始的 HAT 的相对重要性随着从甲基-和苯基环己烷到乙基-、异丙基-和叔-丁基环己烷。在 C-2 处也观察到失活,其位点选择性随着取代基空间体积的增加逐渐转移到 C-3 和 C-4。在乙基(三氟甲基)二环氧乙烷促进的相应氧化中观察到的位点选择性支持这种机制图。将这些结果与先前获得的由苯基和叔的 PINO 自由基促进的 C-H 键叠氮化和官能化的结果进行比较-丁基环己烷与新的计算一起,为理解环烷烃的 C-H 键官能化提供了一个机制框架。HAT 试剂的性质、C-H 键强度DOI:10.1021/acs.joc.1c00902
-
作为产物:描述:2-(5-Bromo-pentylidene)-malonic acid dimethyl ester 以 N,N-二甲基甲酰胺 为溶剂, 以60%的产率得到环戊基丙二酸二甲酯参考文献:名称:电解环化。电化学和类似化学(MIRC)反应的比较摘要:给出了一些说明电还原环化和MIRC反应的对比和互补方面的例子。给出了电化学反应的简要机理讨论,并提供了使用电化学产生的碱的两个例子。DOI:10.1016/s0040-4039(00)87098-7
文献信息
-
[EN] INHIBITORS OF HISTONE DEACETYLASE<br/>[FR] INHIBITEURS D'HISTONE DÉSACÉTYLASE申请人:RODIN THERAPEUTICS INC公开号:WO2015200619A1公开(公告)日:2015-12-30This invention provides compounds that are inhibitors of HDAC2. The compounds (e.g., compounds according to Formula (I), (II), (IIa), (III), (IV), (V), or (VI)) accordingly are useful for treating, alleviating, or preventing a condition in a subject such as a neurological disorder, memory or cognitive function disorder or impairment, extinction learning disorder, fungal disease or infection, inflammatory disease, hematological disease, or neoplastic disease, or for improving memory or treating, alleviating, or preventing memory loss or impairment.这项发明提供了抑制HDAC2的化合物。这些化合物(例如,符合式(I)、(II)、(IIa)、(III)、(IV)、(V)或(VI)的化合物)可用于治疗、缓解或预防受试者的疾病,如神经系统疾病、记忆或认知功能障碍或损伤、消退学习障碍、真菌疾病或感染、炎症性疾病、血液疾病或肿瘤性疾病,或用于改善记忆或治疗、缓解或预防记忆丧失或损伤。
-
Pyrazolopyrimidines申请人:Gebauer Olaf公开号:US20050187224A1公开(公告)日:2005-08-25The invention relates to prazolopyrimidines of the formula in which R 1 , R 2 , R 3 , R 4 , R 5 , and X are as defined in the disclosure, to a process for preparing these compounds, and to their use for controlling unwanted microorganisms.
-
Electrochemical cyclodimerization of alkylidenemalonates作者:Michail N. Elinson、Sergey K. Feducovich、Alexandre A. Zakharenkov、Bogdan I. Ugrak、Gennady I. Nikishin、Sergey V. Lindeman、Jurii T. StruchkovDOI:10.1016/0040-4020(95)98700-r日期:1995.4in MeOH in the presence of alkali metal halide as mediator, leads to the formation of cyclic dimers, i.e., 3,4-disubstituted 1,1,2,2-cyclobutanetetracarboxylates. The reaction proceeds via the reductive coupling of two substrate molecules at cathode and the cyclization of a hydrodimer dianion by its interaction with an active form of a mediator, an anode-generated halogen.
-
POLYMERIZATION INITIATOR, CURABLE COMPOSITION, DENTAL MATERIAL, AND PREPARATION KIT FOR CURABLE COMPOSITION申请人:Mitsui Chemicals, Inc.公开号:EP3835329A1公开(公告)日:2021-06-16Provided is a polymerization initiator, which has an excellent curing rate and is an alternative to a polymerization initiator system using a benzoyl peroxide-aromatic amine compound, a curable composition containing the polymerization initiator, a dental material and dental filler material containing the composition, and a kit for preparing the curable composition. The present invention includes a polymerization initiator containing one or more compounds (A) selected from the group consisting of a pyrazolidinedione compound and/or pyrazolidine(di)thione compound (A1), a salt (A2) of the compound (A1), and a malonate compound (A3).
-
Total synthesis of spatane diterpenes: the tricyclic nucleus作者:Robert G. Salomon、Navzer D. Sachinvala、Subhas Roy、Basudeb Basu、Swadesh R. Raychaudhuri、Donald B. Miller、Ram B. SharmaDOI:10.1021/ja00008a043日期:1991.4A convergent, stereocontrolled construction of the cis,anti,cis-tricyclo[5.3.0.0(2,6)]decane nucleus of spatane diterpenes was achieved by 2-pi + 2-pi photocycloaddition of 2-cyclopenten-1-one, as an A-ring precursor, with a carbonyl-masked derivative of 6-methylbicyclo[2.2.1]hept-5-en-2-one as a temporarily bridged C-ring precursor. By design, the temporary bridge assures the correct stereochemical relationship between the B-ring stereocenters and the C-ring hydroxyl substituent that is present in latent form in the oxoethano bridge. Serendipitously, the bridge also fosters a favorable orientation of the photocycloaddition that contrasts with the nonselective 2-pi + 2-pi-photocycloadditions of unbridged 1-methylcyclopentenes with 2-cyclopenten-1-one. Wittig methylenation of the A-ring carbonyl in photoproduct 24 followed by hydrolysis to 25 and catalytic hydrogenation introduces a methyl group at position 1 with a 10:1 preference for the requisite stereochemistry in 26. Even higher stereoselectivity was achieved by SO2-promoted isomerization of the exocyclic C=C bond in 25 to an endocyclic disposition in 29 prior to catalytic hydrogenation. Johnson's sulfoximine method is especially effective for resolution of ketone 29. The oxoethano bridge in ketone 26n is oxidized rapidly but nonregioselectively by an H2SO4-catalyzed reaction with peracetic acid, producing a 57:43 mixture of 14 and 34. Regioselective generation of the desired lactone 14 could be accomplished by a much slower oxidation with peracetic acid and no added H2SO4. The necessary configuration at position 7 in 49 was generated stereoselectively by a novel homoallylic hydroxyl-directed, pseudointramolecular delivery of hydride to the methylenemalonic ester in the precursor 48. Conversion of the malonic ester moiety in 49 into an allylic alcohol was accomplished in 92% overall yield by monosaponification, decarboxylative condensation with formaldehyde and reduction of the resulting acrylic ester with i-Bu2AlH. Selective oxidation of the allylic hydroxyl followed by acetylation delivered acetate (+)-11a, which is identical with an oxidative degradation product from spatane diterpenes.
表征谱图
-
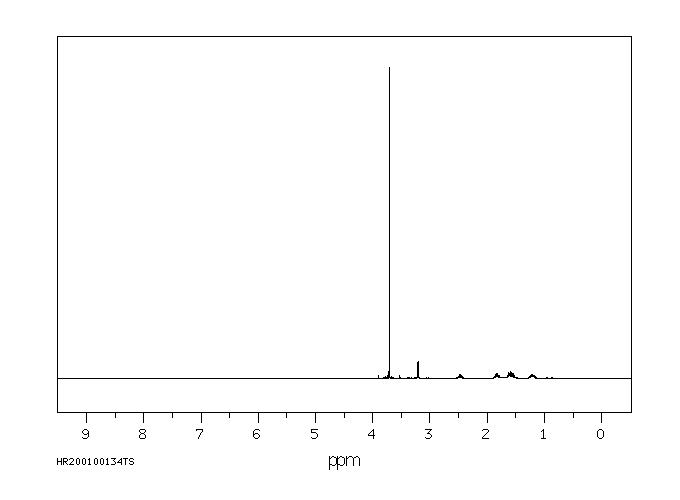
氢谱1HNMR
-
质谱MS
-
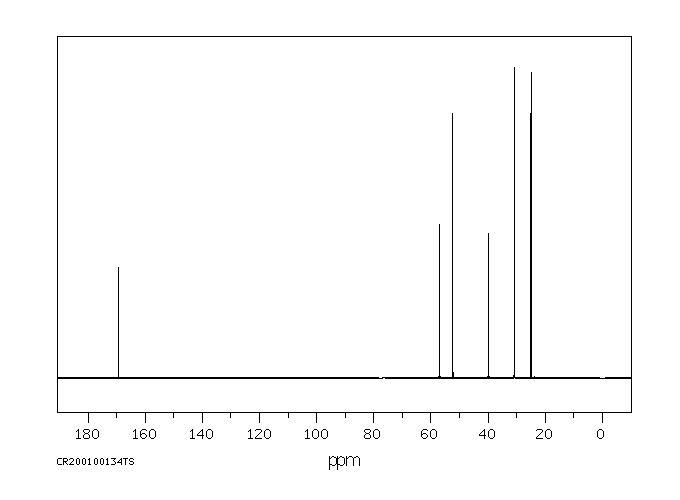
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸