3,5-二(2-吡啶基)吡唑 | 129485-83-2
中文名称
3,5-二(2-吡啶基)吡唑
中文别名
——
英文名称
3,5-di(pyridin-2-yl)-1H-pyrazole
英文别名
3,5-bis(2-pyridyl)pyrazole;3,5-di(2-pyridyl)pyrazole;2,2'-(1H-pyrazole-3,5-diyl)dipyridine;2-(3-pyridin-2-yl-1H-pyrazol-5-yl)pyridine
CAS
129485-83-2
化学式
C13H10N4
mdl
——
分子量
222.249
InChiKey
IMDRKCUYKQQEAC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:188 °C
-
沸点:476.5±35.0 °C(Predicted)
-
密度:1.248±0.06 g/cm3(Predicted)
-
溶解度:在热甲醇中几乎透明
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:17
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:54.5
-
氢给体数:1
-
氢受体数:3
安全信息
-
海关编码:2933990090
-
危险性防范说明:P264,P280,P302+P352+P332+P313+P362+P364,P305+P351+P338+P337+P313
-
危险性描述:H315,H319
SDS
Section I.Chemical Product and Company Identification
Chemical Name 3,5-Di(2-pyridyl)pyrazole
Portland OR
Synonym
Not available.
Chemical Formula
C 13 H 10 N 4
CAS Number
Not available.
(International)
Section II. Composition and Information on Ingredients
Chemical Name CAS Number Percent (%) TLV/PEL Toxicology Data
3,5-Di(2-pyridyl)pyrazole Min. 98.0 (T) Not available. Not available.
Not available.
Section III. Hazards Identification
Acute Health Effects
N o specific inform ation is available in our data base regarding the toxic effects of this m aterial for hum ans. H ow ever,
exposure to any chem ical should be kept to a m inim u m . Skin and eye contact m ay result in irritation. M ay be harm ful if
inhaled or ingested. A lw ays follow safe industrial hygiene practices and w ear proper protective equipm ent w hen
handling this compound.
Chronic Health Effects
CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY : Not available.
There is no know n effect from chronic exposure to this product. Repeated or prolonged exposure to this com pound is
not known to aggravate existing medical conditions.
Section IV. First Aid Measures
Eye Contact
C heck for and rem ove any contact lenses. D O N O T use an eye ointm ent. Flush eyes w ith running w ater for a m inim um
of 15 m inutes, occasionally lifting the upper and low er eyelids. Seek m edical attention. Treat sym ptom atically and
supportively.
Skin Contact
If the chem ical gets spilled on a clothed portion of the body, rem ove the contam inated clothes as quickly as possible,
protectin g you r ow n h an ds an d body. Place th e victim u n der a delu g e sh ow er. If th e ch em ical tou ch es th e victim 's
exposed skin, such as the hands: G ently and thoroughly w ash the contam inated skin w ith running w ater and
non-abrasive soap. Be particularly careful to clean folds, crevices, creases and groin. C over the irritated skin w ith an
em ollient. Seek m edical attention. Treat sym ptom atically and supportively. W ash any contam inated clothing before
reusing.
Inhalation
If the victim is not breathing, perform artificial respiration. Loosen tight clothing such as a collar, tie, belt or w aistband.
If breathing is difficult, oxygen can be administered. Seek medical attention. Treat symptomatically and supportively.
Ingestion
IN D U C E V O M ITIN G by sticking finger in throat. Low er the head so that the vom it w ill not reenter the m outh and throat.
Loosen tight clothing such as a collar, tie, belt, or w aistband. If the victim is not breathing, adm inister artificial
respiration. Exam ine the lips and m outh to ascertain w hether the tissues are dam aged, a possible indication that the
toxic m aterial w as ingested; the absence of such signs, how ever, is not conclusive. Seek im m ediate m edical attention
and, if possible, show the chemical label. Treat symptomatically and supportively.
Section V. Fire and Explosion Data
Auto-Ignition
Flammability Not available.
Combustible.
Flash Points Flammable Limits
Not available.
Not available.
Combustion Products
These products are toxic carbon oxides (CO, CO 2), nitrogen oxides (NO, NO 2 ).
Fire Hazards
No specific information is available regarding the flammability of this compound in the presence of various materials.
Explosion Hazards
Risks of explosion of the product in presence of mechanical impact: Not available.
Risks of explosion of the product in presence of static discharge: Not available.
No additional information is available regarding the risks of explosion.
Fire Fighting Media
SMALL FIRE: Use DRY chemicals, CO 2, water spray or foam.
and Instructions LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet.
Continued on Next Page
Not a 3,5-Di(2-pyridyl)pyrazole
Section VI. Accidental Release Measures
Spill Cleanup
In case of a spill and/or a leak, alw ays shut off any sources of ignition, ventilate the area, and exercise caution. U se a
Instructions shovel to put the m aterial into a convenient w aste disposal container. Finish cleaning the spill by rinsing any
contam inated surfaces w ith copious am ounts of w ater. C onsult federal, state, and/or local authorities for assistance on
disposal.
Section VII. Handling and Storage
Handling and Storage
K eep aw ay from heat and sources of ignition. M echanical exhaust required. W hen not in use, tightly seal the container
and store in a dry, cool place. A void excessive h eat and light. D O N O T breathe dust. In case of in sufficient ventilation,
Information
w ear suitable respiratory equipm ent. If you feel u nw ell, seek m edical attention and show the label w h en possible. Treat
symptomatically and supportively. Avoid contact with skin and eyes.
Always store away from incompatible compounds such as oxidizing agents.
Section VIII. Exposure Controls/Personal Protection
Engineering Controls
U se process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below
recom m ended exposure lim its. If user operations generate dust, fum e or m ist, use ventilation to keep exposure to
airborne contaminants below the exposure limit.
Personal Protection
Splash goggles. Lab coat. D ust respirator. Boots. G loves. A M SH A /N IO SH approved respirator m ust be used to avoid
inhalation of the product. Suggested protective clothing m ight not be sufficient; consult a specialist BEFO RE handling
this product.
Exposure Limits
Not available.
Section IX. Physical and Chemical Properties
Physical state @ 20°C Solubility
White needle-like crystals.
Not available.
Specific Gravity
Not available.
Molecular Weight Partition Coefficient
222.25 Not available.
Boiling Point Vapor Pressure
Not available. Not available.
Melting Point Vapor Density
188°C (370.4°F) Not available.
Refractive Index Volatility
Not available. Not available.
Critical Temperature Odor
Not available. Not available.
Viscosity Taste
Not available. Not available.
Section X. Stability and Reactivity Data
Stability
This material is stable if stored under proper conditions. (See Section VII for instructions)
Conditions of Instability
Avoid excessive heat and light.
Incompatibilities
Reactive with oxidizing agents.
Section XI. Toxicological Information
RTECS Number
Not available.
Routes of Exposure
Eye contact. Inhalation. Ingestion.
Toxicity Data
Not available.
Chronic Toxic Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY : Not available.
There is no know n effect from chronic exposure to this product. Repeated or prolonged exposure to this com pound is
not known to aggravate existing medical conditions.
Acute Toxic Effects
N o specific inform ation is available in our data base regarding the toxic effects of this m aterial for hum ans. H ow ever,
exposure to any chem ical should be kept to a m inim u m . Skin and eye contact m ay result in irritation. M ay be harm ful if
inhaled or ingested. A lw ays follow safe industrial hygiene practices and w ear proper protective equipm ent w hen
handling this compound.
Continued on Next Page
3,5-Di(2-pyridyl)pyrazole
Section XII. Ecological Information
Ecotoxicity
Not available.
Environmental Fate
Not available.
Section XIII. Disposal Considerations
Waste Disposal Recycle to process, if possible. C onsult your local or regional authorities. You m ay be able to dissolve or m ix m aterial
w ith a com bustible solvent and burn in a chem ical incinerator equipped w ith an afterburner and scrubber system .
Observe all federal, state, and local regulations when disposing of this substance.
Section XIV. Transport Information
DOT Classification
Not a DOT controlled material (United States).
PIN Number
Not applicable.
Proper Shipping Name
Not applicable.
Packing Group (PG)
Not applicable.
DOT Pictograms
Section XV. Other Regulatory Information and Pictograms
TSCA Chemical Inventory
T h is p r o d u c t is NOT o n t h e E P A T o x ic S u b s t a n c e s C o n t r o l A c t (T S C A ) in v e n t o r y . T h e f o llo w in g n o t ic e s a r e r e q u ir e d b y 4 0
(EPA) CFR 720.36 (C) for those products not on the inventory list:
(i) These products are supplied solely for use in research and developm ent by or under the supervision of a technically
qualified individual as defined in 40 CFR 720.0 et sec.
(ii) The health risks of these products have not been fully determ ined. A ny inform ation that is or becom es available w ill
be supplied on an MSDS sheet.
WHMIS Classification
Not controlled under WHMIS (Canada).
(Canada)
EINECS Number (EEC)
Not available.
EEC Risk Statements
Not available.
Japanese Regulatory Data Not available.
Section XVI.
Version 1.0
Validated on 5/16/1997.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4-bromo-3,5-bis(2-pyridyl)-1H-pyrazole 922506-47-6 C13H9BrN4 301.145 —— 4-chloro-3,5-bis(2-pyridyl)-1H-pyrazole 922506-46-5 C13H9ClN4 256.694 —— 4-iodo-3,5-bis(2-pyridyl)-1H-pyrazole 922506-48-7 C13H9IN4 348.146 —— 4-nitro-3,5-bis(2-pyridyl)-1H-pyrazole 922506-49-8 C13H9N5O2 267.247
反应信息
-
作为反应物:描述:3,5-二(2-吡啶基)吡唑 在 碘 、 sodium carbonate 、 potassium iodide 作用下, 以 二氯甲烷 为溶剂, 反应 4.0h, 以98%的产率得到4-iodo-3,5-bis(2-pyridyl)-1H-pyrazole参考文献:名称:合成一些新的4-取代-3,5-双(2-吡啶基)-1 h-吡唑摘要:在温和的反应条件下,以高收率合成了几种4-取代的3,5-双(2-吡啶基)-1 H-吡唑,其中的取代基是氯,溴,碘,硝基,重氮。产物的结构通过1 H NMR,13 C NMR,ESI-MS,IR和元素分析进行表征。DOI:10.1002/jhet.5570430637
-
作为产物:描述:参考文献:名称:用于有机发光二极管的天蓝色铱(III)配合物的高效电致发光摘要:合成了两种新型的天蓝色铱(III)配合物Ir(dfppy)2 pypzpy和Ir(dfppy)2 phpzpy,其中2-(2,4-二氟苯基)吡啶(dfppy)被用作主要配体,2,2引入'-(1 H-吡唑-3,5-二基)二吡啶(pypzpy)和2-(3-2-(3-苯基-1 H-吡唑-5-基)吡啶(phpzpy)作为辅助配体两种Ir(III)配合物在469和472 nm处均表现出相似的发射峰,分别具有0.82和0.86的高量子效率;此外,具有ITO / HATCN(六氮杂三苯六碳六烯)结构的有机发光二极管(OLED), 5 nm)/ TAPC(双[4-(N,N-ditolylamino)-phenyl]环己烷,40 nm)/ mCP(1,3-双(9 H-咔唑-9-基)苯,5 nm)/ Ir(dfppy)2 pypzpy或Ir(dfppy)2 phpzpy(10 wt%):PPO21(3-(二苯DOI:10.1016/j.dyepig.2018.06.011
文献信息
-
Mechanistic Insight into Synergistic Catalysis of Olefin Hydrogenation by a Hetero-Dinuclear Ru<sup>II</sup>–Co<sup>II</sup> Complex with Adjacent Reaction Sites作者:Dachao Hong、Yuji Ohgomori、Yoshihiro Shimoyama、Hiroaki Kotani、Tomoya Ishizuka、Yoshihiro Kon、Takahiko KojimaDOI:10.1021/acs.inorgchem.9b02104日期:2019.9.3have designed and synthesized a hetero-dinuclear RuII–CoII complex with a dinucleating ligand inspired by hetero-dinuclear active sites of metalloenzymes. A synergistic effect between the adjacent RuII and CoII sites has been confirmed in catalytic olefin hydrogenation by the complex, exhibiting a much higher turnover number than those of mononuclear RuII or CoII complexes as the components. A RuII-hydrido
-
Seven Reversible Redox Processes in a Self-Assembled Cobalt Pentanuclear Bis(triple-stranded helicate): Structural, Spectroscopic, and Magnetic Characterizations in the Co<sup>I</sup>Co<sup>II</sup><sub>4</sub>, Co<sup>II</sup><sub>5</sub>, and Co<sup>II</sup><sub>3</sub>Co<sup>III</sup><sub>2</sub> Redox States作者:Eric Gouré、Bertrand Gerey、Florian Molton、Jacques Pécaut、Rodolphe Clérac、Fabrice Thomas、Jérôme Fortage、Marie-Noëlle CollombDOI:10.1021/acs.inorgchem.0c01102日期:2020.7.6potential range, highlighting the remarkable stability of such architecture in several redox states. Two mixed-valent states of this complex, the two-electron-oxidized CoII3CoIII2 (15+) and the one-electron-reduced species CoICoII4 (12+), were generated by bulk electrolyses and successfully characterized by single-crystal X-ray diffraction among the eight redox levels between CoI5 and CoII3CoIII2 that我们报道了刚性四齿双(2-吡啶基)-3,5-吡唑酸酯配体BPP – [Co II(μ-BPP)3 } 2 Co II的五核钴螺旋复合物的合成和结构表征3(μ 3 -OH)] 3+(1 3+),其中三核钴II 3(μ 3 -OH)}芯由两个钴包裹II(μ-BPP)3 }单位。CH 3中1 3+的循环伏安图CN揭示了在0和-3.0 V电位范围内的七个连续的可逆单电子波,突显了这种体系结构在几种氧化还原状态下的出色稳定性。通过本体电解生成该络合物的两个混合价态,即二电子氧化的Co II 3 Co III 2(1 5+)和单电子还原的Co I Co II 4(1 2+)。并成功地通过Co I 5和Co II 3 Co III 2之间的八个氧化还原水平之间的单晶X射线衍射表征可以在电化学条件下访问。由于1 5+和1 2+的晶体学特征,五个还原过程分别位于E 1/2值为-1.63(1 3+ / 2
-
Highly efficient green electroluminescence of iridium(<scp>iii</scp>) complexes based on (1<i>H</i>-pyrazol-5-yl)pyridine derivatives ancillary ligands with low efficiency roll-off作者:Ning Su、Guang-Zhao Lu、You-Xuan ZhengDOI:10.1039/c8tc01182f日期:——displayed high current efficiency with low efficiency roll-off. Moreover, the device based on the Ir-me complex exhibited the best performances with a maximum luminance of 38 155 cd m−2, maximum current efficiency of 92 cd A−1, and a maximum external quantum efficiency of 28.90%. These results suggested that green Ir(III) complexes were obtained by modification of the ppy ligand and rational introduction of合成了铱(Ir-me),Ir-cf3,Ir-py和Ir-ph四种铱(III)配合物,其中2-(4-三氟甲基)苯基吡啶(tfmppy)被用作主要配体,而2- (3-甲基-1 H-吡唑-5-基)吡啶(mepzpy),2-(3-(三氟甲基)-1 H-吡唑-5-基)吡啶(cf3pzpy),2,2'-(1 H -吡唑-3,5-二基)联吡啶(pypzpy)和2-(3-苯基-1 H-吡唑-5-基)吡啶(phpzpy)分别用作辅助配体。所有复合物在494–499 nm处均显示出类似的绿光峰,并具有较高的磷光量子效率(0.76-0.82)。具有ITO / HATCN(六氮杂三亚苯基六碳三烯)(5 nm)/ TAPC(双[4-(N,N-二糖基氨基)-苯基]环己烷,50 nm)/ Ir络合物的结构的有机发光二极管(OLED)(8 wt%):TCTA(4,4',4''-三(9-咔唑基)三苯胺,20 nm)/ TmPyPB(1
-
A Bpp-based dinuclear ruthenium photocatalyst for visible light-driven oxidation reactions作者:Seán Hennessey、Pau Farràs、Jordi Benet-Buchholz、Antoni LlobetDOI:10.1039/c9cy01796h日期:——out-/in-[(bpy)2Ru(bpp)Ru(L)(tpy)]n+ (bpy = 2,2′-bipyridine, tpy = 2,2′:6′,2′′-terpyridine, L = Cl, CF3COO−, H2O or CH3CN and n = 2 or 3) has been prepared and fully characterised. The complex has been characterized by analytical and spectroscopic techniques and by X-ray diffraction analysis for two of the derivatives (Cl and CH3CN). Additionally, full electrochemical characterization based on cyclic voltammetry and由2,2-(1 H-吡唑-3,5-二基)二吡啶(Hbpp)桥联配体组成的二钌二联分子,分子式为out- / in-[(bpy)2 Ru(bpp)Ru(L) (TPY)] ñ +(BPY = 2,2'-联吡啶,TPY = 2,2':6',2'' -三联吡啶,L =氯,CF 3 COO -,H 2 O或CH 3 CN和ñ = 2或3)已经准备好并充分表征。该配合物已通过分析和光谱技术以及X射线衍射分析对其中两种衍生物(Cl和CH 3CN)。另外,还进行了基于循环伏安法和方波伏安法的完全电化学表征。还研究了氧化还原对对水基络合物的pH依赖性,并绘制了相应的Pourbaix图。此外,已经进行了光催化氧化有机底物如醇,烯烃和硫化物的能力,并且已经分析了催化剂的整体稳定性和选择性。
-
GLUCOKINASE ACTIVATORS申请人:Cao X. Sheldon公开号:US20070197532A1公开(公告)日:2007-08-23Compounds, pharmaceutical compositions, kits and methods are provided for use with glucokinase that comprise a compound selected from the group consisting of: wherein the variables are as defined herein.提供了用于与葡萄糖激酶一起使用的化合物、药物组合物、试剂盒和方法,其中包括从以下组中选择的化合物:其中变量如本文所定义。
表征谱图
-
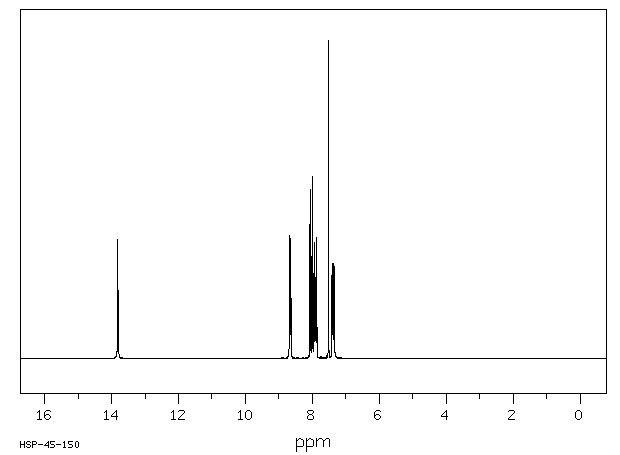
氢谱1HNMR
-
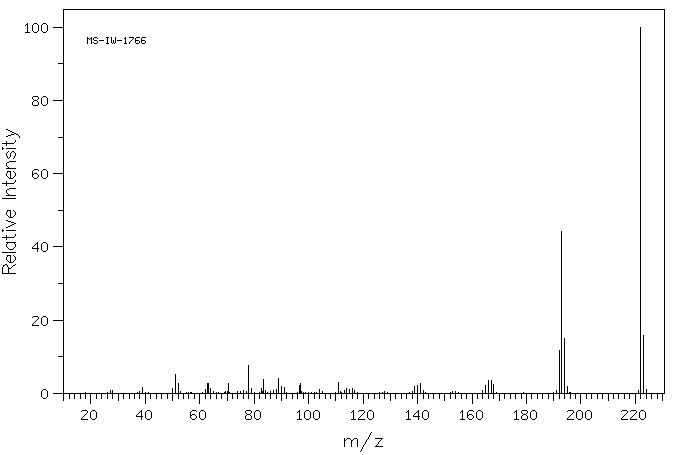
质谱MS
-
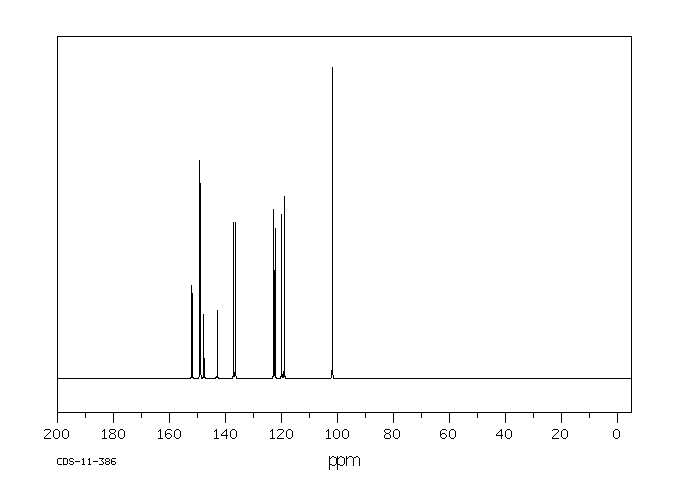
碳谱13CNMR
-
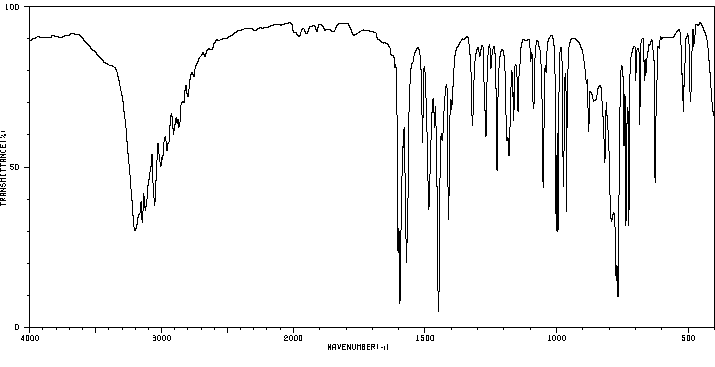
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-