2,4,5-三(2-吡啶基)咪唑 | 23974-92-7
中文名称
2,4,5-三(2-吡啶基)咪唑
中文别名
——
英文名称
2,4,5-tris(2-pyridyl)imidazole
英文别名
2,2',2''-(1H-imidazole-2,4,5-triyl)tripyridine;2,4,5-Tris-<2>pyridyl-imidazol;2,4,5-Tris(2-pyridyl)-imidazol;2,2',2''-(1H-imidazole-2,4,5-triyl)-tris-pyridine;2,4,5-Tris(2-pyridyl)imidazole;2-(2,4-dipyridin-2-yl-1H-imidazol-5-yl)pyridine
CAS
23974-92-7
化学式
C18H13N5
mdl
——
分子量
299.335
InChiKey
FCCAYZJCTJTONV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:558.1±45.0 °C(Predicted)
-
密度:1.265±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:23
-
可旋转键数:3
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:67.4
-
氢给体数:1
-
氢受体数:4
反应信息
-
作为反应物:描述:2,4,5-三(2-吡啶基)咪唑 、 copper dichloride 以 乙醇 为溶剂, 生成 C18H12Cu2N5(3+)参考文献:名称:Application of 2,4,5‐tris(2‐pyridyl)imidazole as ‘turn‐off’ fluorescence sensor for Cu(II) and Hg(II) ions and in vitro cell imaging摘要:2,4,5-三(2-吡啶基)咪唑(L)分子已被评估为在乙醇/HEPES缓冲液(5mM,pH=7.34,1:1,v/v)中用于汞2+和铜2+离子双重传感的探针。探针L在汞2+和铜2+离子同时存在的情况下显示出良好的灵敏度和选择性关闭响应,这在长紫外线照射下是可以理解的。该探针可以在pH值3-11范围内检测铜2+离子,在pH值6-8范围内检测汞2+离子。铜2+的检测限(0.77μM)远低于美国环境保护署规定的允许限值。每升溶液需要两种金属离子(铜2+和汞2+)才能完全淬灭荧光。该探针在用乙二胺四乙酸钠处理时显示出明显的可逆性,使该方案在实际应用中更加经济。用乙醇L溶液涂覆的纸条可以通过荧光强度的可见淬灭来检测样品中铜2+和汞2+离子的存在。密度泛函理论-时间依赖密度泛函理论(DFT-TDDFT)计算支持实验观察,铜2+/汞2+的d轨道提供了非辐射衰变途径。使用HDF和MDA-MB-2DOI:10.1002/bio.4232
-
作为产物:参考文献:名称:Hydrogenation of heteroaromatic nitriles and aromatic dinitriles by heterogeneous or homogeneous ruthenium catalysts derived from [Ru 3 (CO) 12 ]摘要:The use of the complex [Ru-3(CO)(12)] (1) as a catalyst precursor (0.1 mol%) at 200 degrees C, 60 psi of H-2, along with triphenylphosphine (TPP) generated ruthenium nanoparticles (Ru-Nps); this occurred in the presence of pyridine-nitriles leading to a variety of hydrogenation (secondary amine, imine, or imidazole) products, depending of the pyridine-nitrile used, under similar reaction conditions. This relates to relatively good to modest yields, determined by the substituents in the corresponding pyridine. In sharp contrast, the use of aromatic dinitriles did not generate Ru-Nps at 140 degrees C, 150 psi of H-2 and TPP, but allowed the homogeneous catalytic hydrogenation of the 1,4- and 1,3-dicyanobenzenes, to yield the corresponding CN-substituted secondary amine or imine. The main products were characterized by different analytical methods and spectroscopic techniques. (C) 2017 Elsevier B.V. All rights reserved.DOI:10.1016/j.ica.2017.04.051
文献信息
-
Novel synthesis of 2,4-bis(2-pyridyl)-5-(pyridyl)imidazoles and formation of N-(3-(pyridyl)imidazo[1,5-a]pyridine)picolinamidines: nitrogen-rich ligands作者:Vijendra Kumar Fulwa、Rojalin Sahu、Himanshu Sekhar Jena、Vadivelu ManivannanDOI:10.1016/j.tetlet.2009.09.002日期:2009.115-tris(2-pyridyl)imidazole (1a) as the major product and N-(3-(2-pyridyl)imidazo[1,5-a]pyridine)picolinamidine (2a) in small amounts. Similarly, by using 3-picolylamine, 2,4,-bis(2-pyridyl)-5-(3-pyridyl)imidazole (1b) and N-(3-(3-pyridyl)imidazo[1,5-a]pyridine)picolinamidine (2b) were isolated, and by using 4-picolylamine, 2,4,-bis(2-pyridyl)-5-(4-pyridyl)imidazole (1c) and N-(3-(4-pyridyl)imidazo[1,5加热纯的2-picolylamine和2-cyanopyridine的1:2混合物,然后用KOH水溶液处理所得的红色胶状物质,分离出2,4,5-tris(2-吡啶基)咪唑(1a)作为主要产物和少量N-(3-(2-吡啶基)咪唑并[1,5- a ]吡啶)吡啶啉am(2a)。类似地,通过使用3-甲基吡啶胺,2,4,-双(2-吡啶基)-5-(3-吡啶基)咪唑(1b)和N-(3-(3-吡啶基)咪唑[1,5- a ]分离吡啶)吡啶啉am(2b),并使用4-吡啶甲基胺,2,4,-双(2-吡啶基)-5-(4-吡啶基)咪唑(1c)和N-(3-(4-吡啶基)咪唑[1,5-分离出[ ]吡啶)吡啶啉am(2c)。描绘了1a – c和2a – c形成的合理机制。
表征谱图
-
氢谱1HNMR
-
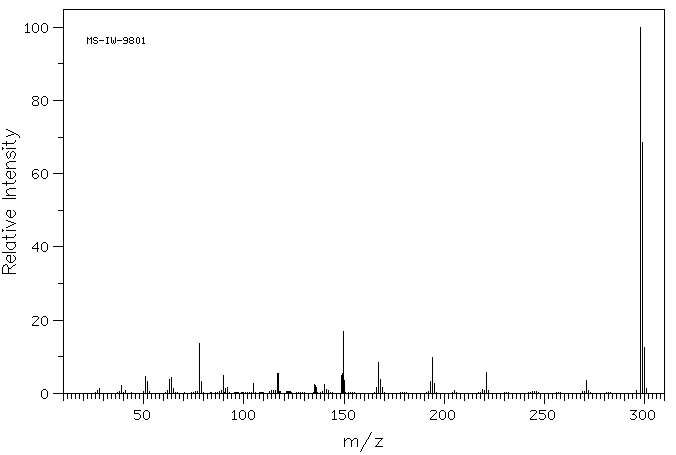
质谱MS
-
碳谱13CNMR
-
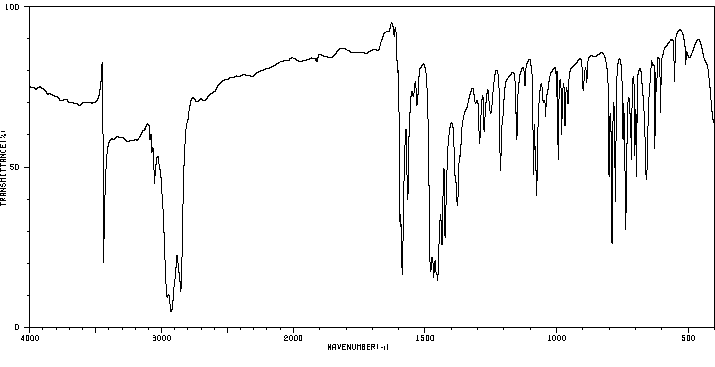
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(SP-4-1)-二氯双(1-苯基-1H-咪唑-κN3)-钯
(5aS,6R,9S,9aR)-5a,6,7,8,9,9a-六氢-6,11,11-三甲基-2-(2,3,4,5,6-五氟苯基)-6,9-甲基-4H-[1,2,4]三唑[3,4-c][1,4]苯并恶嗪四氟硼酸酯
(5-氨基-1,3,4-噻二唑-2-基)甲醇
齐墩果-2,12-二烯[2,3-d]异恶唑-28-酸
黄曲霉毒素H1
高效液相卡套柱
非昔硝唑
非布索坦杂质Z19
非布索坦杂质T
非布索坦杂质K
非布索坦杂质E
非布索坦杂质D
非布索坦杂质67
非布索坦杂质65
非布索坦杂质64
非布索坦杂质61
非布索坦代谢物67M-4
非布索坦代谢物67M-2
非布索坦代谢物 67M-1
非布索坦-D9
非布索坦
非唑拉明
雷非那酮-d7
雷西那德杂质2
雷西纳德杂质L
雷西纳德杂质H
雷西纳德杂质B
雷西纳德
雷西奈德杂质
阿西司特
阿莫奈韦
阿考替胺杂质9
阿米苯唑
阿米特罗13C2,15N2
阿瑞匹坦杂质
阿格列扎
阿扎司特
阿尔吡登
阿塔鲁伦中间体
阿培利司N-1
阿哌沙班杂质26
阿哌沙班杂质15
阿可替尼
阿作莫兰
阿佐塞米
镁(2+)(Z)-4'-羟基-3'-甲氧基肉桂酸酯
锌1,2-二甲基咪唑二氯化物
锌(II)(苯甲醇)(四苯基卟啉)
锌(II)(正丁醇)(四苯基卟啉)
锌(II)(异丁醇)(四苯基卟啉)