7-乙基-1,3,5-环庚三烯 | 17634-51-4
中文名称
7-乙基-1,3,5-环庚三烯
中文别名
——
英文名称
7-ethyl-1,3,5-cycloheptatriene
英文别名
7-Aethyl-cycloheptatrien;ethylcycloheptatriene;7-ethylcycloheptatriene;7-Ethyl-cycloheptatrien;7-ethylcyclohepta-1,3,5-triene;7-Aethyl-1,3,5-cycloheptatrien;7-Ethyl-1,3,5-cycloheptatrien
CAS
17634-51-4
化学式
C9H12
mdl
——
分子量
120.194
InChiKey
DKCPRYXLHFWDCQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:55-57 °C(Press: 22 Torr)
-
密度:0.848±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:9
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.33
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 2-cyclohepta-2,4,6-trienyl-ethanol 70186-84-4 C9H12O 136.194
反应信息
-
作为反应物:描述:7-乙基-1,3,5-环庚三烯 以36%的产率得到参考文献:名称:ASAO TOYONOBU; YAGIHARA MORIO; KITAHARA YOSHIO, HETEROCYCLES, 1981, 15, NO 2, SPEC. ISSUE, 985-991摘要:DOI:
-
作为产物:参考文献:名称:Isomerization of Alkyl Tropilidenes1摘要:DOI:10.1021/ja01471a027
文献信息
-
Catalytic [6π+2π]-cycloaddition of Si-containing alkynes to 7-substituted 1,3,5-cycloheptatrienes under the action of Ti(acac)2Cl2–Et2AlCl作者:Vladimir A. D'yakonov、Gulnara N. Kadikova、Dmitriy I. Kolokol'tsev、Ilfir R. Ramazanov、Usein M. DzhemilevDOI:10.1016/j.jorganchem.2015.06.006日期:2015.10two-component Ti(acac)2Cl2–Et2AlCl system, resulting in the formation of substituted bicyclo[4.2.1]nona-2,4,7-trienes in high yields, was accomplished. The structures of the obtained compounds were confirmed by 1H, 13C NMR spectroscopy. The reaction mechanism was studied through quantum chemical modeling of the cycloheptatriene and acetylene interaction with TiCl4–Et2AlCl system at the B3LYP/6-31G(d) level
-
Titanium-Catalyzed [6π+2π]-Cycloaddition of Alkynes and Allenes to 7-Substituted 1,3,5-Cycloheptatrienes作者:Vladimir A. D'yakonov、Gulnara N. Kadikova、Dmitry I. Kolokol'tsev、Ilfir R. Ramazanov、Usein M. DzhemilevDOI:10.1002/ejoc.201500442日期:2015.75-cycloheptatrienes with alkynes and allenes, catalyzed by the two-component Ti(acac)2Cl2–Et2AlCl system, resulting in the formation of substituted bicyclo[4.2.1]nona-2,4-dienes and bicyclo[4.2.1]nona-2,4,7-trienes in up to 90 % yield, was accomplished. The structures of the obtained compounds were confirmed by X-ray diffraction and by 1H and 13C NMR spectroscopic analysis.
-
Regioselective [6π+2π] cycloaddition of 1,2-dienes to 7-substituted 1,3,5-cycloheptatrienes catalyzed by Ti(acac)2Cl2—Et2AlCl作者:G. N. Kadikova、D. I. Kolokoltsev、E. S. Meshcheryakova、V. A. D’yakonov、U. M. DzhemilevDOI:10.1007/s11172-016-1283-5日期:2016.1A reaction of 7-alkyl-, 7-allyl-, 7-phenyl-1,3,5-cycloheptatrienes with 1,2-dienes in the presence of the two-component catalytic system Ti(acac)2Cl2—Et2AlCl, which led to the formation of practically important substituted endo-bicyclo[4.2.1]nona-2,4-dienes in up to 90% yields, was accomplished for the first time.在双组分催化体系 Ti(acac)2Cl2-Et2AlCl 的存在下,7-烷基-、7-烯丙基-、7-苯基-1,3,5-环庚三烯与 1,2-二烯首次发生反应,生成了实际上重要的取代内双环[4.2.1]壬-2,4-二烯,收率高达 90%。
-
One-Electron Reduction of Carbonium Ions. IV. A Kinetic Study on the Reduction of the Substituted Tropylium Ions with Cr(II)作者:Kunio Okamoto、Koichi Komatsu、Osamu Murai、Osamu Sakaguchi、Yoshihisa MatsuiDOI:10.1246/bcsj.46.1785日期:1973.6unsubstituted, methyl-, ethyl-, isopropyl-, t-butyl-, triphenylmethyl-, and phenyltropylium ions with Cr(II) in a 10% HCl solution gives, quantitatively, the dimer of the corresponding substituted tropyl radical. The measurements of the reaction rate at 25°C exhibit this order of reactivity: t-butyltropylium (k2=7.98 l/g·ion·sec), isopropyltropylium (8.22), ethyltropylium (10.3), methyltropylium (11.1), tropylium在 10% HCl 溶液中用 Cr(II) 对未取代的甲基-、乙基-、异丙基-、叔丁基-、三苯基甲基-和苯基鎓离子进行单电子还原,定量得到相应取代的二聚体托自由基。25°C 下反应速率的测量显示出以下反应性顺序:t-丁基托鎓 (k2=7.98 l/g·离子·秒)、异丙基鎓 (8.22)、乙基托鎓 (10.3)、甲基托鎓 (11.1)、托鎓 (74.0) )、苯基鎓 (144) 和三苯基甲基鎓 (567) 离子。log k2 的值与观察到的这些碳正离子与芘的电荷转移带的跃迁能以及相应阳离子的极谱半波电位呈线性相关。这些相关性表明碳正离子在与 Cr(II) 还原中的反应性主要由各自阳离子中固有的电子亲和力决定。log k2 的值也与 pKR+ 值具有良好的线性相关性,这意味着...
-
Reactions of Cyclic C<sub>7</sub>H<sub>6</sub>-System: Reactions of a Tautomer of Cycloheptatetraene and Cycloheptatrienylidene with Tropone, Heptafulvene, and 1,3,5-Cycloheptatriene Derivatives作者:Katsuhiro Saito、Satoshi Suzuki、Tetsuya Watanabe、Kensuke TakahashiDOI:10.1246/bcsj.66.2304日期:1993.8Reactions of a cyclic C7H6-system, a tautomer of 1,2,4,6-cycloheptatetraene and 2,4,6-cycloheptatrienylidene, with tropone derivatives and 8,8-dicyanoheptafulvene afforded exo-[4π+2π] cycloadducts. Similar reactions with 1,3,5-cycloheptatriene derivatives gave exo-[6π+2π] cycloadducts. These reactions are considered to proceed through zwitter ionic intermediates containing tropylium ion moieties.
表征谱图
-
氢谱1HNMR
-
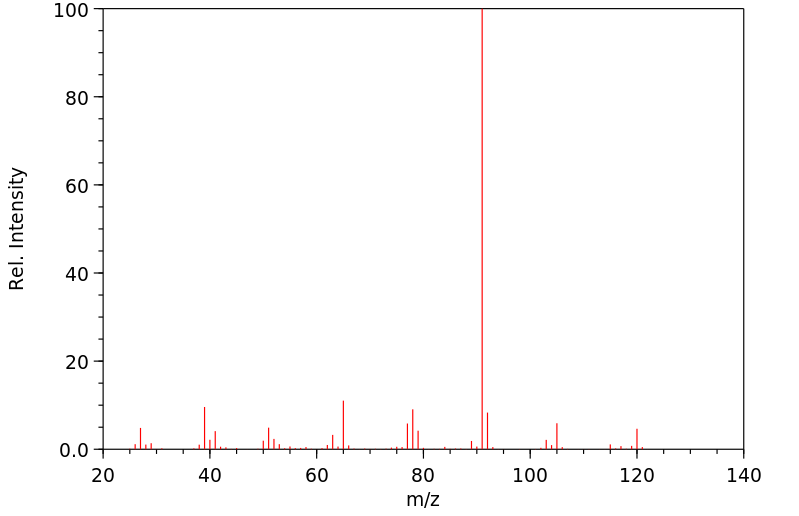
质谱MS
-
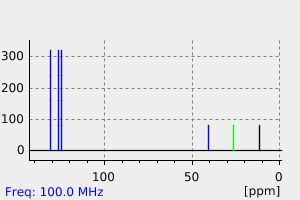
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-