5-己烯-3-酮 | 24253-30-3
中文名称
5-己烯-3-酮
中文别名
——
英文名称
5-hexen-3-one
英文别名
1-Hexen-4-on;allyl ethyl ketone;Hex-5-en-3-on;hex-5-en-3-one
CAS
24253-30-3
化学式
C6H10O
mdl
——
分子量
98.1448
InChiKey
RUJLJMUWUVTHEU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:124.0-124.2 °C
-
密度:0.84976 g/cm3
-
LogP:1.244 (est)
-
稳定性/保质期:
存在于主流烟气中。
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:7
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2914190090
SDS
反应信息
-
作为反应物:描述:5-己烯-3-酮 在 RhCl(PPh3)3 rhodium(II) octanoate 、 4-乙酰氨基苯磺酰叠氮 、 氢气 、 sodium cyanoborohydride 、 1,8-二氮杂双环[5.4.0]十一碳-7-烯 、 三氟乙酸 作用下, 以 乙醇 、 正己烷 、 二氯甲烷 、 水 、 乙腈 为溶剂, 25.0 ℃ 、310.27 kPa 条件下, 反应 14.25h, 生成 8-methyl-2-propanoyl-8-azabicyclo<3.2.1>oct-2-ene参考文献:名称:Synthesis of 2.beta.-Acyl-3.beta.-aryl-8-azabicyclo[3.2.1]octanes and Their Binding Affinities at Dopamine and Serotonin Transport Sites in Rat Striatum and Frontal Cortex摘要:A novel entry to tropane analogs of cocaine was developed on the basis of the reaction of rhodium-stabilized vinylcarbenoids with pyrroles. These analogs were tested:in binding to dopamine and serotonin (5-HT) transporters in:membranes from rat striatum and frontal cortex. In all the analogs, the aryl group at the 3-position was directly bound to the tropane ring (as in WIN-35,428), and methyl or ethyl ketone moieties were present at the a-position instead of the typical ester group. The series of analogs containing a 2-naphthyl group at the 3-position were most potent, with K-i values < 1 nM in binding to both dopamine and 5-HT transporters. Although the unsubstituted 2-naphthyl analog was nonselective at dopamine and 5-HT transport sites, other compounds:were selective for either site. In general, compounds with relatively small substituents on the aromatic moiety (such as p-methyl or p-fluoro) were relatively selective for the dopamine transporters, while a p-isopropylphenyl derivative was selective:for the 5-HT transport sites. This latter compound represents the first N-methyltropane derivative specific for 5-HT transporters. Resolution of two of the most significant analogs was achieved by HPLC on a chiral stationary phase; the active enantiomer of a 2-naphthyl analog exhibited K-i values of <0.1 nM at both dopamine and 5-HT transporter sites.DOI:10.1021/jm00035a005
-
作为产物:参考文献:名称:Mkryan,G.M. et al., Journal of Organic Chemistry USSR (English Translation), 1969, vol. 5, p. 1524 - 1526摘要:DOI:
文献信息
-
The Cinchona Primary Amine-Catalyzed Asymmetric Epoxidation and Hydroperoxidation of α,β-Unsaturated Carbonyl Compounds with Hydrogen Peroxide作者:Olga Lifchits、Manuel Mahlau、Corinna M. Reisinger、Anna Lee、Christophe Farès、Iakov Polyak、Gopinadhanpillai Gopakumar、Walter Thiel、Benjamin ListDOI:10.1021/ja402058v日期:2013.5.1Using cinchona alkaloid-derived primary amines as catalysts and aqueous hydrogen peroxide as the oxidant, we have developed highly enantioselective Weitz-Scheffer-type epoxidation and hydroperoxidation reactions of α,β-unsaturated carbonyl compounds (up to 99.5:0.5 er). In this article, we present our full studies on this family of reactions, employing acyclic enones, 5-15-membered cyclic enones, and
-
Expedient Synthesis of 1,5-Diketones by Rhodium-Catalyzed Hydroacylation Enabled by C–C Bond Cleavage作者:Rui Guo、Guozhu ZhangDOI:10.1021/jacs.7b05427日期:2017.9.20A rhodium-catalyzed intermolecular hydroacylation reaction of vinyl cyclobutanols with non-chelating aldehydes has been developed. This reaction offers a new and atom-economical approach for the selective preparation of 1,5-diketones in high yields. Experimental data suggest a sequential ring-opening, transfer hydrogenation, and hydroacylation mechanism. We propose that aldehyde decarbonylation is
-
Cleavage Reactions of Alkoxy Radicals Produced by Anodic Oxidation of<i>t</i>-Alcohols作者:Kazuhiro Maruyama、Katsuya MurakamiDOI:10.1246/bcsj.41.1401日期:1968.6oxidation of t-alcohols in water - dioxane - sodium hydroxide system has been examined. The possibility of production of t-alkoxy radicals by anodic oxidation of t-alcohols in the electrolytic system was found. The relative ease of bond scission of the intermediate t-alkoxy radical was determined by quantitative analysis of the mixture of ketones produced. The relative rates of cleavage decrease in the order:
-
Improved synthesis of β,X-unsaturated ketones by the reaction of allylic zinc bromides with nitriles.
-
ELECTROPHILIC REACTION OF ALLYLTRIMETHYLSILANE WITH NITRILES IN THE PRESENCE OF BORON TRICHLORIDE作者:Hiroshi Hamana、Tsutomu SugasawaDOI:10.1246/cl.1985.921日期:1985.7.5Allyltrimethylsilane reacted with various nitriles in the presence of boron trichloride, giving after hydrolysis β,γ-unsaturated ketones in high yields. The reactions of substituted allyltrimethylsilanes and intramolecular reaction of allylic trimethylsilane with nitrile were also studied.
表征谱图
-
氢谱1HNMR
-
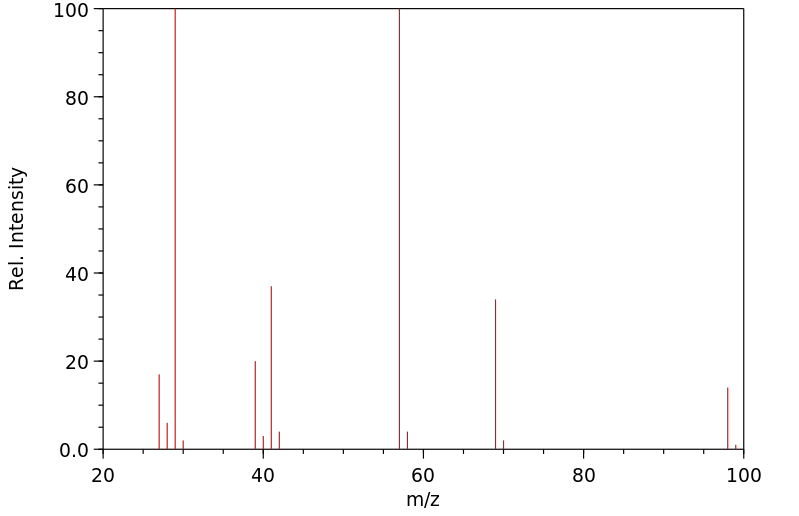
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷