7-十四醇 | 3981-79-1
中文名称
7-十四醇
中文别名
7-十四烷醇
英文名称
7-tetradecanol
英文别名
tetradecan-7-ol;Tetradecanol-(7)
CAS
3981-79-1
化学式
C14H30O
mdl
——
分子量
214.392
InChiKey
FFAQYGTZIMVBFJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:42 °C
-
沸点:156 °C / 14mmHg
-
密度:0.8097 (estimate)
-
保留指数:1666
-
稳定性/保质期:
- 常温常压下稳定。
- 存在于烟气中。
计算性质
-
辛醇/水分配系数(LogP):5.9
-
重原子数:15
-
可旋转键数:11
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
储存条件:请将药品存放在避光、通风干燥的地方,并密封保存。
SDS
7-十四醇 修改号码:6
模块 1. 化学品
产品名称: 7-Tetradecanol
修改号码: 6
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 7-十四醇
百分比: ....
CAS编码: 3981-79-1
分子式: C14H30O
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
7-十四醇 修改号码:6
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点:
42°C
沸点/沸程 156 °C/1.9kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
7-十四醇 修改号码:6
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
7-十四醇 修改号码:6
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 7-Tetradecanol
修改号码: 6
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 7-十四醇
百分比: ....
CAS编码: 3981-79-1
分子式: C14H30O
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
7-十四醇 修改号码:6
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点:
42°C
沸点/沸程 156 °C/1.9kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
7-十四醇 修改号码:6
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
7-十四醇 修改号码:6
模块16 - 其他信息
N/A
上下游信息
反应信息
-
作为反应物:参考文献:名称:IONIZABLE CATIONIC LIPID FOR RNA DELIVERY摘要:描述的是一种化合物,其化学式为I,包括以下成分: R1为由10至31个碳组成的支链烷基化合物; R2为由2至20个碳组成的直链烷基、烯基或炔基; L1和L2相同或不同,每个为由1至20个碳组成的直链烷基或由2至20个碳组成的直链烯基; X1为S或O; R3为由1至6个碳组成的直链或支链烷基; R4和R5相同或不同,每个为氢或由1至6个碳组成的直链或支链烷基;或其药用可接受盐。公开号:US20180169268A1
-
作为产物:参考文献:名称:金属卟啉催化剂的形状选择性烷烃羟基化摘要:一系列具有空间保护口袋的锰和铁卟啉被证明是形状选择性烷烃羟基化催化剂。以碘代苯作为氧化剂,通过使用空间位阻非常大的 (5,10,15,20-四(2',4',6'-三苯基)-卟啉基),在最小受阻甲基处观察到烷烃的羟基化具有良好的区域选择性乙酸锰(III)(MnTTPPP(OAc))作为催化剂;中等受阻的(5,10,15,20-四(2',4',6'-三甲氧基苯基)卟啉)锰(III)醋酸盐对末端 CH/sub 3/羟基化几乎没有选择性,但对相邻的, omega-1, CH/sub 2/ 位点。初级选择性取决于烷烃底物的大小和形状,更大的取代基会产生更大的初级选择性。取代五氟碘代苯或间氯过苯甲酸作为氧化剂产生相似的选择性,因此最终证明通过这三个系统的共同中间体进行基于金属的氧化。相比之下,叔丁基过氧化氢或 2,2,2-三氟乙醇溶解的五氟碘代苯没有伯碳选择性,反应产物比例与金属卟啉催化剂无关;这表明DOI:10.1021/ja00283a024
文献信息
-
Iron‐Catalyzed Wacker‐type Oxidation of Olefins at Room Temperature with 1,3‐Diketones or Neocuproine as Ligands**作者:Florian Puls、Philipp Linke、Olga Kataeva、Hans‐Joachim KnölkerDOI:10.1002/anie.202103222日期:2021.6.14Herein, we describe a convenient and general method for the oxidation of olefins to ketones using either tris(dibenzoylmethanato)iron(III) [Fe(dbm)3] or a combination of iron(II) chloride and neocuproine (2,9-dimethyl-1,10-phenanthroline) as catalysts and phenylsilane (PhSiH3) as additive. All reactions proceed efficiently at room temperature using air as sole oxidant. This transformation has been
-
Catalytic Acceptorless Dehydrogenation of Aliphatic Alcohols作者:Hiromu Fuse、Harunobu Mitsunuma、Motomu KanaiDOI:10.1021/jacs.0c00123日期:2020.3.4aliphatic secondary alcohols to ketones under visible light irradiation at room temperature by devising a ternary hybrid catalyst system comprising a photoredox catalyst, a thiophosphate organocatalyst, and a nickel catalyst. The reaction proceeded through three main steps: hydrogen atom transfer from the α-C-H bond of an alcohol substrate to the thiyl radical of the photo-oxidized organocatalyst, interception
-
Site-Selective Ni-Catalyzed Reductive Coupling of α-Haloboranes with Unactivated Olefins作者:Shang-Zheng Sun、Marino Börjesson、Raul Martin-Montero、Ruben MartinDOI:10.1021/jacs.8b09425日期:2018.10.10A mild, chemo- and site-selective catalytic protocol that allows for incorporating an alkylboron fragment into unactivated olefins is described. The use of internal olefins enables C-C bond-formation at remote sp3 C-H sites, constituting a complementary and conceptually different approach to existing borylation techniques that are currently available at sp3 centers.
-
Titanocenes as Photoredox Catalysts Using Green‐Light Irradiation作者:Zhenhua Zhang、Tobias Hilche、Daniel Slak、Niels R. Rietdijk、Ugochinyere N. Oloyede、Robert A. Flowers、Andreas GansäuerDOI:10.1002/anie.202001508日期:2020.6.8Irradiation of Cp2TiCl2 with green light leads to electronically excited [Cp2TiCl2]*. This complex constitutes an efficient photoredox catalyst for the reduction of epoxides and for 5‐exo cyclizations of suitably unsaturated epoxides. To the best of our knowledge, our system is the first example of a molecular titanium photoredox catalyst.
-
The evaluation of dicyclopentadienylsamarium as a reagent in organic synthesis作者:J.L. Namy、J. Collin、J. Zhang、H.B. KaganDOI:10.1016/s0022-328x(00)99769-9日期:1987.7SmCp2, which is easily prepared from SmI2, has been screened as a reducing agent for organic chemistry. In particular, SmCp2 promotes the pseudo-Barbier reaction between carbonyl compounds (aldehydes and ketones) and aliphatic or allylic halides more efficiently than does SmI2.容易从SmI 2制备的SmCp 2已被筛选为有机化学的还原剂。特别是,SmCp 2比SmI 2更有效地促进羰基化合物(醛和酮)与脂族或烯丙基卤化物之间的拟Barbier反应。
表征谱图
-
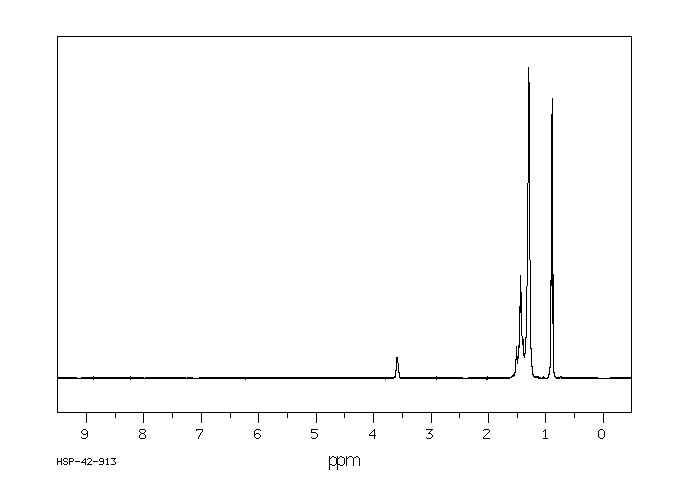
氢谱1HNMR
-
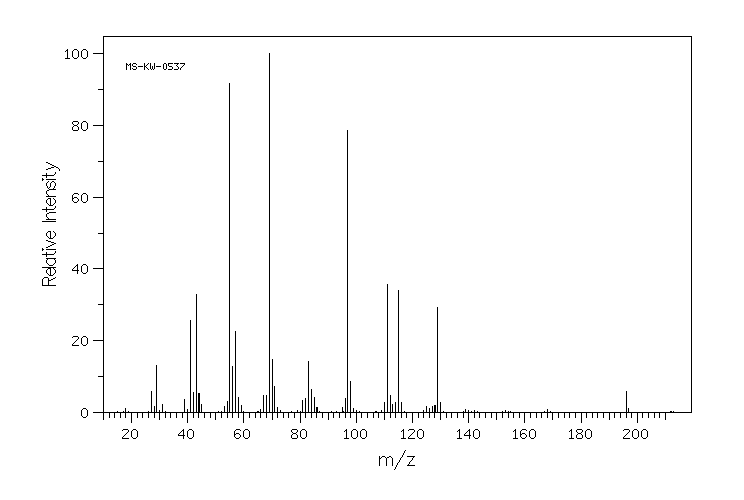
质谱MS
-
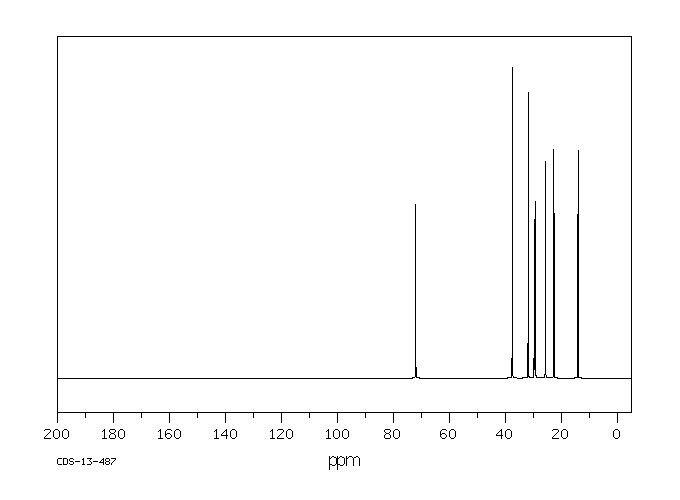
碳谱13CNMR
-
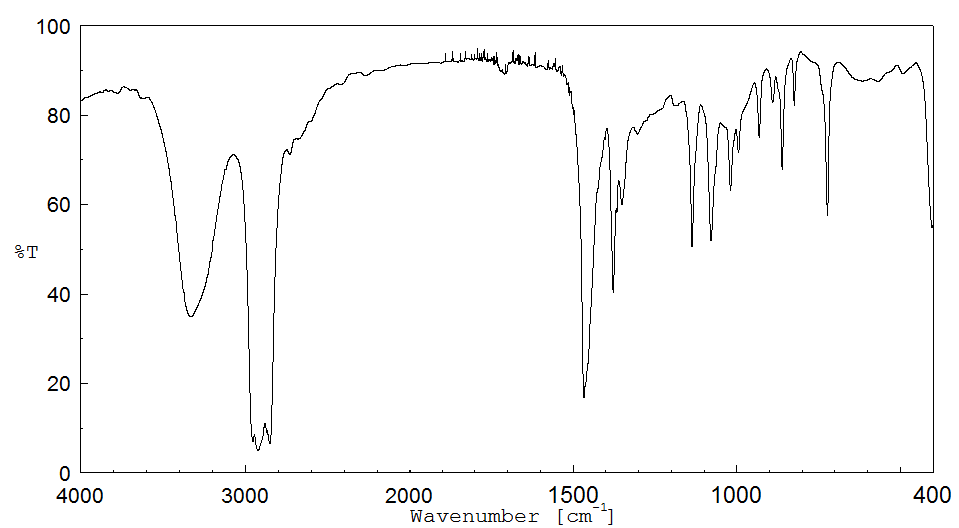
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯