2,3,6-三甲氧基苯甲酸 | 60241-74-9
中文名称
2,3,6-三甲氧基苯甲酸
中文别名
——
英文名称
2,3,6-trimethoxybenzoic acid
英文别名
2,3,6-Trimethoxybenzoesaeure
CAS
60241-74-9
化学式
C10H12O5
mdl
MFCD00092587
分子量
212.202
InChiKey
NSKSXYUOXAQHCH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:150°C
-
沸点:344.5±37.0 °C(Predicted)
-
密度:1.219±0.06 g/cm3(Predicted)
-
稳定性/保质期:
避免接触氧化物
计算性质
-
辛醇/水分配系数(LogP):0.6
-
重原子数:15
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:65
-
氢给体数:1
-
氢受体数:5
安全信息
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
海关编码:2918990090
-
储存条件:在密封的贮藏器中,应放置于阴凉、干燥处并保持良好通风。
SDS
2,3,6-三甲氧基苯甲酸 修改号码:5
模块 1. 化学品
产品名称: 2,3,6-Trimethoxybenzoic Acid
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2,3,6-三甲氧基苯甲酸
百分比: >98.0%(GC)(T)
CAS编码: 60241-74-9
分子式: C10H12O5
2,3,6-三甲氧基苯甲酸 修改号码:5
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
2,3,6-三甲氧基苯甲酸 修改号码:5
模块 9. 理化特性
熔点:
150°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
2,3,6-三甲氧基苯甲酸 修改号码:5
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 2,3,6-Trimethoxybenzoic Acid
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2,3,6-三甲氧基苯甲酸
百分比: >98.0%(GC)(T)
CAS编码: 60241-74-9
分子式: C10H12O5
2,3,6-三甲氧基苯甲酸 修改号码:5
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
2,3,6-三甲氧基苯甲酸 修改号码:5
模块 9. 理化特性
熔点:
150°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
2,3,6-三甲氧基苯甲酸 修改号码:5
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 6-羟基-2,3-二甲氧基苯甲酸 6-hydroxy-2,3-dimethoxy-benzoic acid 5653-53-2 C9H10O5 198.175 —— methyl 2,3,6-trimethoxybenzoate 60241-75-0 C11H14O5 226.229 —— 2,3,6-Trihydroxy-benzoesaeure 16534-78-4 C7H6O5 170.122 —— 1-(2,3,6-trimethoxy-phenyl)-ethanone 13909-60-9 C11H14O4 210.23 —— 2,3,6-trimethoxytoluene 25576-97-0 C10H14O3 182.219 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— methyl 2,3,6-trimethoxybenzoate 60241-75-0 C11H14O5 226.229 —— ethyl 2,3,6-trimethoxybenzoate —— C12H16O5 240.256 3-氯-2,5,6-三甲氧基苯甲酸 3-chloro-2,5,6-trimethoxybenzoic acid 101460-24-6 C10H11ClO5 246.647 3-溴-2,5,6-三甲氧基苯甲酸 3-bromo-2,5,6-trimethoxybenzoic acid 101460-22-4 C10H11BrO5 291.098 —— 2,3,6-Trihydroxy-benzoesaeure 16534-78-4 C7H6O5 170.122 —— Methyl-2,3,6-trihydroxybenzoat 61885-19-6 C8H8O5 184.149 2,3,6-三甲氧基苯甲酰氯 2,3,6-Trimethoxybenzoesaeurechlorid 58093-61-1 C10H11ClO4 230.648 —— 4-isopropyl-2,3,5-trimethoxybenzyl alcohol 156723-06-7 C13H20O4 240.299 —— 4-isopropyl-2,3,5-trimethoxybenzaldehyde 866464-91-7 C13H18O4 238.284 —— 2-(2,3,6-trimethoxyphenyl)propan-2-ol 60241-76-1 C12H18O4 226.273 —— 2,3,6-trimethoxy-5-nitrobenzoic acid 185316-83-0 C10H11NO7 257.2 —— 3-(1-methylethyl)-1,2,4-trimethoxybenzene 60241-78-3 C12H18O3 210.273 - 1
- 2
反应信息
-
作为反应物:描述:2,3,6-三甲氧基苯甲酸 在 lithium hydroxide 、 magnesium 作用下, 以 四氢呋喃 、 乙醚 为溶剂, 反应 10.0h, 生成 2-(2,3,6-trimethoxyphenyl)propan-2-ol参考文献:名称:通过分子内Heck反应立体选择性合成(±)-Komaroviquinone和(±)-Faveline甲醚摘要:通过分子内Heck反应实现了构建反式-10-羟基-1,1-二甲基八氢二苯并[ a,d ]环庚-7-酮(5a - c)的有效,灵活和立体选择性的聚合途径。该策略已成功实施,用于通过(±)-香豆酮二甲醚(5c)和(±)-faveline甲醚(1a)合成(±)-科马罗维醌(3)。DOI:10.1021/jo051082i
-
作为产物:描述:参考文献:名称:ROTENONE. XIII. OXIDATION OF METHYLDERRITOLIC ACID AND THE SYNTHESIS OF 2,3,5- AND 2,3,6-TRIMETHOXYBENZOIC ACIDS AND THEIR DERIVATIVES摘要:DOI:10.1021/ja01359a033
文献信息
-
[EN] PENICILLIN-BINDING PROTEIN INHIBITORS<br/>[FR] INHIBITEURS DE PROTÉINE DE LIAISON À LA PÉNICILLINE申请人:VENATORX PHARMACEUTICALS INC公开号:WO2019226931A1公开(公告)日:2019-11-28Described herein are certain boron-containing compounds, compositions, preparations and their use as modulators of the transpeptidase function of bacterial penicillin-binding proteins and as antibacterial agents. In some embodiments, the compounds described herein inhibit penicillin-binding proteins. In certain embodiments, the compounds described herein are useful in the treatment of bacterial infections.
-
Synthesis of polyhydroxylated flavonoids bearing a lipophilic decyl tail as potential therapeutic antioxidants作者:Stuart T. Caldwell、Donald B. McPhail、Garry G. Duthie、Richard C. HartleyDOI:10.1139/v11-087日期:2012.1
Antioxidants have potential for the treatment of stroke and neurodegeneration, and chimeric compounds that combine a flavon-3-ol head group related to myricetin and a lipophilic decyl tail are known to protect membranes from oxidative damage at least as well as vitamin E. New flavon-3-ols that are highly hydroxylated in the B ring in ways not found in natural flavon-3-ols and bearing a lipophilic decyl tail have been prepared from trimethoxy- and tetramethoxybenzoic acids accessed by lithiation–carboxylation reactions. Direct enolate acylation was preferred over Baker–Venkataraman rearrangement when there were methoxy groups at both the 2- and the 6-position of the benzoic acid derivatives.
-
Preparation of aromatic and heteroaromatic carboxylic acids by catalytic ozonolysis申请人:——公开号:US20030216577A1公开(公告)日:2003-11-20A process for catalytically oxidizing alkylaromatic compounds of the formula (I) Ar—CH 2 —R where Ar is an optionally substituted, aromatic or heteroaromatic 5-membered or 6-membered ring or a ring system having up to 20 carbon atoms where Ar may optionally be fused to a C 1 -C 6 -alkyl group in which up to 2 carbon atoms may be replaced by a heteroatom, and R is hydrogen, phenyl, benzyl or heteroaryl, where the phenyl, benzyl or heteroaryl radicals may also be joined to Ar by a bridge, or R together with Ar forms an optionally substituted ring system which may contain one or more optionally substituted heteroatoms, to the corresponding aromatic or heteroaromatic carboxylic acids in a solvent with ozone in the presence of a transition metal catalyst and optionally in the presence of an acid at a temperature between −70° C. and 110° C. to the corresponding carboxylic acid.
-
1,3,5,8-四羟基呫吨酮和1,3,7,8-四羟基呫吨酮的制备方法申请人:广州药本君安医药科技股份有限公司公开号:CN110156739A公开(公告)日:2019-08-23本发明公开了1,3,5,8‑四羟基呫吨酮和1,3,7,8‑四羟基呫吨酮的制备方法,该方法包括以下步骤:以1,2,4‑三甲氧基苯为起始原料,加入正丁基锂和氯甲酸酯反应,生成2,3,6‑三甲氧基苯甲酸甲酯;加入碱反应,生成2,3,6‑三甲氧基苯甲酸;加入均苯三酚,再加入伊顿试剂反应,生成1,3‑二羟基‑5(7),8‑二甲氧基呫吨酮异构体混合物;最后加入试剂A反应,生成1,3,5,8‑四羟基呫吨酮和1,3,7,8‑四羟基呫吨酮异构体混合物,经过分离,得到产物。本发明中1,3,5,8‑四羟基呫吨酮和1,3,7,8‑四羟基呫吨酮的制备方法,具有反应条件温和、合成试剂环境友好、步骤简洁、反应总收率较高的特点。
-
Development of potent inhibitors for strigolactone receptor DWARF 14作者:Masahiko Yoshimura、Sojung F. Kim、Ryosuke Takise、Shuhei Kusano、Sakuya Nakamura、Masanori Izumi、Akiko Yagi、Kenichiro Itami、Shinya HagiharaDOI:10.1039/d0cc01989e日期:——Strigolactones (SLs) are plant hormones that suppress shoot branching through perception by their receptor protein DWARF 14 (D14). The artificial regulation of SL signaling has been considered a potent agricultural technique because plant architecture is strongly related to crop yield. In this communication, we describe the development of a small-molecule D14 inhibitor that functions at sub-micromolar
表征谱图
-
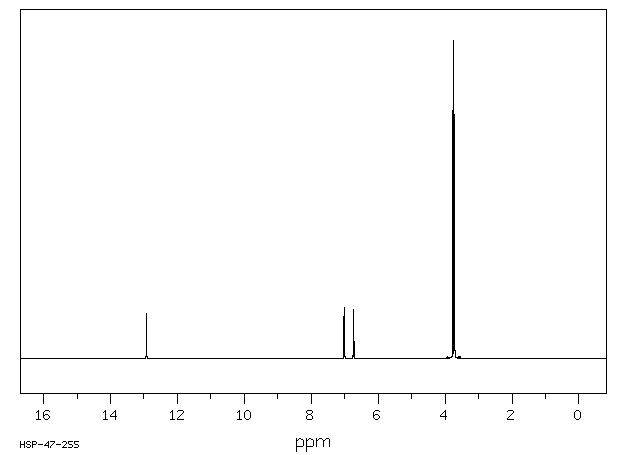
氢谱1HNMR
-
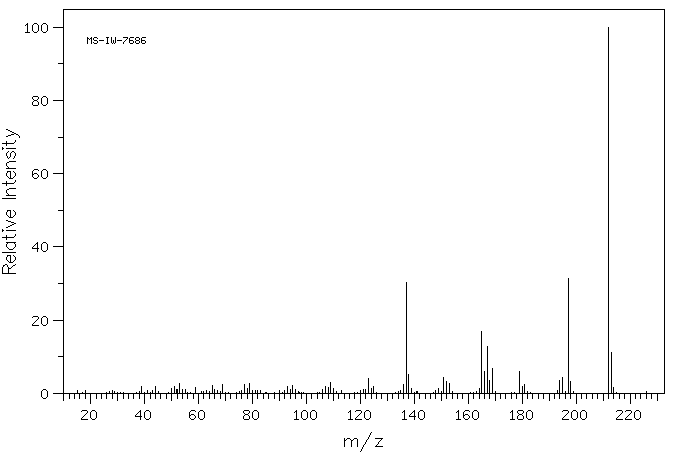
质谱MS
-
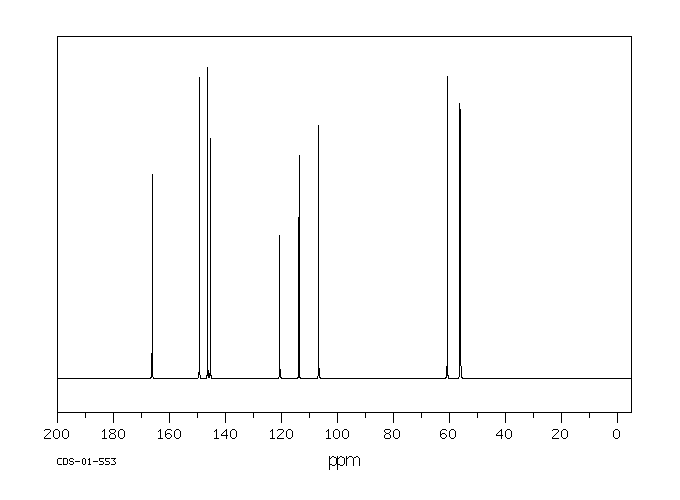
碳谱13CNMR
-
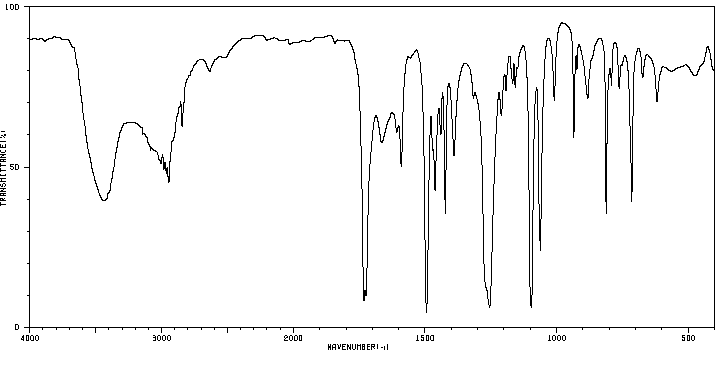
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫