4,10-二氧杂三环[5.2.1.0(2,6)]癸-8-烯-3,5-二酮 | 5426-09-5
中文名称
4,10-二氧杂三环[5.2.1.0(2,6)]癸-8-烯-3,5-二酮
中文别名
4,10-二氧杂三环[5.2.1.02,6]癸-8-烯-3,5-二酮;氧杂酸酐;3a,4,7,7a-四氢-4,7-环氧异苯并呋喃-1,3-二酮
英文名称
7-oxanorborn-5-ene-2,3-dicarboxylic anhydride
英文别名
3a,4,7,7a-tetrahydro-4,7-epoxyisobenzofuran-1,3-dione;4,10-dioxatricyclo[5.2.1.02,6]dec-8-ene-3,5-dione
CAS
5426-09-5
化学式
C8H6O4
mdl
MFCD00151506
分子量
166.133
InChiKey
QQYNRBAAQFZCLF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:118 °C
-
沸点:372.0±42.0 °C(Predicted)
-
密度:1.540±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):-0.5
-
重原子数:12
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:52.6
-
氢给体数:0
-
氢受体数:4
安全信息
-
海关编码:2932999099
-
储存条件:2-8°C,储存在惰性气体中。
SDS
| Name: | 3 6-Endoxo-1 2 3 6-tetrahydrophthalic anhydride 98% Material Safety Data Sheet |
| Synonym: | 7-Oxabicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic anhydrid |
| CAS: | 5426-09-5 |
Synonym:7-Oxabicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic anhydrid
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 5426-09-5 | 3,6-Endoxo-1,2,3,6-tetrahydrophthalic | 98.0 | 226-570-2 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.The toxicological properties of this material have not been fully investigated.Moisture sensitive.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation.
Ingestion:
May cause digestive tract disturbances. The toxicological properties of this substance have not been fully investigated.
Inhalation:
Causes respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Causes irritation of mucous membrane.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Do not induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Antidote: None reported.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Do NOT use water directly on fire. In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Keep containers tightly closed.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 5426-09-5: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white - white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 118 deg C dec
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H8O3
Molecular Weight: 166.13
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures. May decompose on exposure to moist air or water.
Conditions to Avoid:
Incompatible materials, dust generation, strong oxidants, exposure to moist air or water.
Incompatibilities with Other Materials:
Strong oxidizing agents - strong acids - strong bases.
Hazardous Decomposition Products:
Hydrogen chloride, phosgene, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 5426-09-5 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3,6-Endoxo-1,2,3,6-tetrahydrophthalic anhydride - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 5426-09-5: No information available.
Canada
CAS# 5426-09-5 is listed on Canada's NDSL List.
CAS# 5426-09-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 5426-09-5 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4,10-二氧杂三环[5.2.1.0(2,6)]癸-8-烯-3-酮 4,10-dioxatricyclo[5.2.1.02,6]deca-8-en-3-one 72150-22-2 C8H8O3 152.15 —— (1R,2S,3R,4S)-3-(methoxycarbonyl)-7-oxabicyclo[2.2.1]hept-5-ene-2-carboxylic acid 104485-82-7 C9H10O5 198.175 —— 5,6-didehydronorcantharidin monomethyl ester —— C9H10O5 198.175 —— 5,6-bis(methoxycarbonyl)-7-oxabicyclo<2,2,1>-2-heptene 59275-03-5 C10H12O5 212.202 —— 5-ene-norcantharidin mono benzyl ester —— C15H14O5 274.273 —— 3-Carbamoyl-7-oxabicyclo[2.2.1]hept-5-ene-2-carboxylic acid 57957-89-8 C8H9NO4 183.16 去甲斑蝥素 endothall anhydride 5442-12-6 C8H8O4 168.149 —— <5,5-(2)H2>-4,10-dioxatricyclo<5.2.1.02.6>dec-8-en-3-one 96995-04-9 C8H8O3 154.134 —— 7-oxabicyclo[2.2.1]heptane-2,3-dicarboxylic acid monoethyl ester 115122-59-3 C10H14O5 214.218 —— 9-oxotricyclo<4.2.1.02,5>nonane-3,4,7,8-tetracarboxylic dianhydride 20220-81-9 C12H8O7 264.191 7-氧杂双环[2.2.1]庚烷-2,3-二甲酸单 2-甲酯 norcantharidin monomethyl ester 57105-58-5 C9H12O5 200.191 —— exo-2,3-bis(hydroxymethyl)-7-oxabicyclo[2.2.1]hept-5-ene 817201-06-2 C8H12O3 156.181 —— endo,endo-7-oxabicyclo[2.2.1]hept-5-ene-2,3-dimethanol 105761-77-1 C8H12O3 156.181 —— 5,6-bis-hydroxymethyl-7-oxa-bicyclo[2.2.1]hept-2-ene 115888-24-9 C8H12O3 156.181 5,6-二溴-7-氧杂双环[2.2.1]庚烷-2,3-二甲酸酐 5,6-dibromohexahydro-4,7-epoxyisobenzofuran-1,3-dione 51371-59-6 C8H6Br2O4 325.941 —— 3-(1,3-Thiazol-2-ylcarbamoyl)-7-oxabicyclo[2.2.1]hept-5-ene-2-carboxylic acid 5694-84-8 C11H10N2O4S 266.28 —— 7-oxabicyclo[2.2.1]heptane-2,3-dicarboxylic acid, mono (phenylmethyl)ester 140486-19-7 C15H16O5 276.289 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:一种催化呋喃和顺酐合成邻苯二甲酸酐的方法摘要:本发明属于生物基化学品技术领域,具体涉及一种催化呋喃和顺酐合成邻苯二甲酸酐的方法。本发明一种催化呋喃和顺酐生成苯酐的制备方法是通过使用呋喃和顺酐为原料,采用Diels‑Alder反应和脱水步骤生产苯酐,其中,所述的呋喃和顺酐是由生物质转化的糠醛中获得。采用本发明方法绿色经济且可再生,减少了不可再生传统资源的使用,为生物基苯酐的工业化生产提供先进的技术支持,具有广阔的应用前景。公开号:CN111892562B
-
作为产物:参考文献:名称:Photopolymerisation process with two exposures of a single layer摘要:一层液态组合物,其中含有一种化合物(A),该化合物在同一分子中至少具有一个(甲基)丙烯酰基团和至少一个双环[2.2.1]庚-2-烯-6-基单元,该层暴露于光致辐射中,以使该层由于(A)通过(甲基)丙烯酰基团的光聚合而固化,但仍保持光交联性。当需要时,将固化的层暴露于较大量的光致辐射中,所进一步暴露的部分通过双环[2.2.1]庚-2-烯-6-基单元更高度光交联,因此不溶。通过适当的溶剂可以产生图像。 (A)的示例包括3-(甲基丙烯酰氧基)-2-羟基丙基双环[2.2.1]庚-2-烯-6-羧酸酯和2-(丙烯酰氧基)乙基甲基-5-羧基双环[2.2.1]庚-2-烯-6-羧酸酯。公开号:US04440850A1
文献信息
-
HEPATITIS C VIRUS INHIBITORS AND USES THEREOF IN PREPARATION OF DRUGS申请人:CHANGZHOU YINSHENG PHARMACEUTICAL CO., LTD.公开号:US20170253614A1公开(公告)日:2017-09-07A series of hepatitis C virus (HCV) inhibitors and compositions and applications thereof in the preparation of drugs for treating chronic HCV infection. Especially, a series of compounds that are used as NS5A inhibitors, and compositions and uses thereof in the preparations of drugs.
-
Universal building blocks and support media for synthesis of oligonucleotides and their analogs申请人:——公开号:US20040152905A1公开(公告)日:2004-08-05Compounds for the synthesis of oligomeric compounds, particularly oligonucleotides and chemically modified oligonucleotide analogs, are provided. In addition, methods for functionalization of a support medium with a first monomeric subunit and methods for the synthesis of oligomeric compounds utilizing the novel compounds bound to support media are provided.提供用于合成寡聚化合物,特别是寡核苷酸和化学修饰的寡核苷酸类似物的化合物。此外,还提供了将支持介质功能化为第一单体亚基的方法,以及利用与支持介质结合的新化合物合成寡聚化合物的方法。
-
喜树碱-甘氨酸-5,6-双脱氢去甲斑蝥素结合物及其应用
-
喜树碱-甘氨酸-5,6-二溴去甲斑蝥素结合物及其应用
-
喜树碱衍生物及其抗肿瘤应用
表征谱图
-
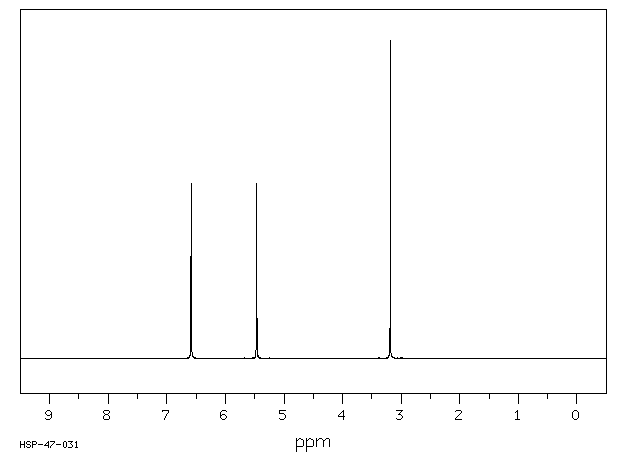
氢谱1HNMR
-
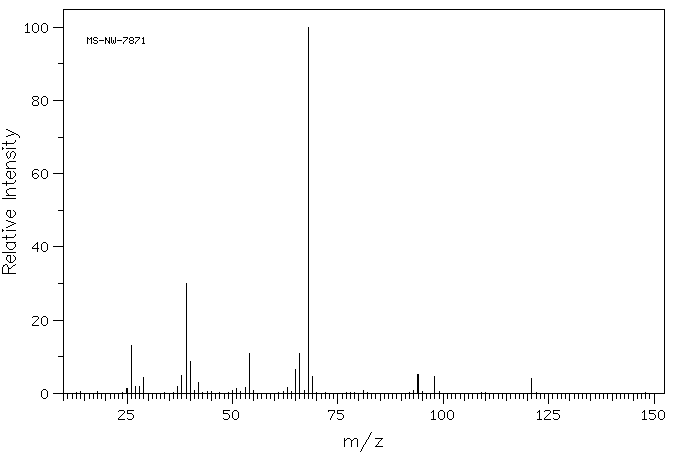
质谱MS
-
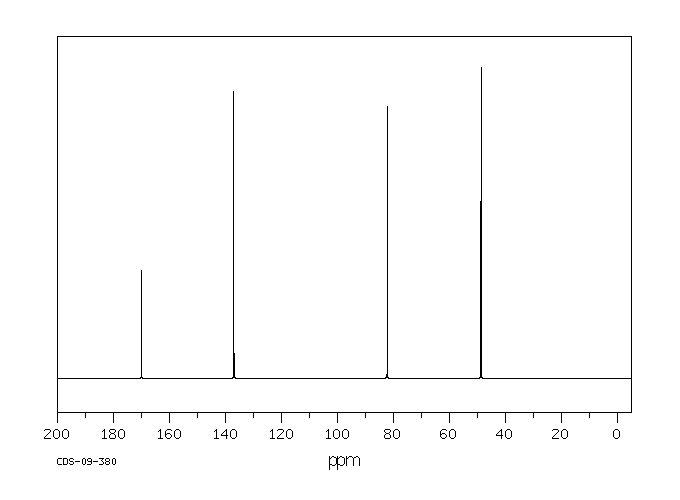
碳谱13CNMR
-
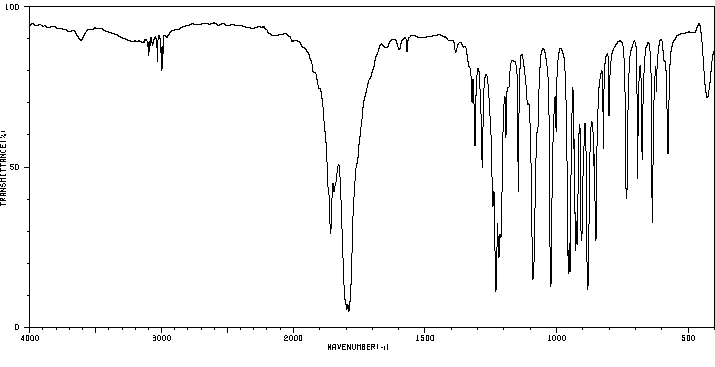
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-2,3,3a,6a-四氢呋喃[2,3-b]呋喃
莱克酮
索尼地平
硝酸异山梨酯
溴化二氢6-(联苯基-4-基)-3-氯-12,13-二甲氧基-9,10--7H-异奎并[2,1-d][1,4]苯并二氮卓-8-正离子
星形曲霉毒素
抗坏血酸原 A
异山梨醇二甲基醚
异山梨醇13C65-单酸酯
异山梨醇
失水甘露醇单油酸酯
失水甘露醇单油酸酯
大青素
地瑞那韦中间体1
四氢呋喃[2,3-B]呋喃-2(6AH)-酮
四氢-6a-甲基-呋喃并[2,3-b]呋喃-2(3H)-酮
四氢-6-硫代-1,4-乙桥-1H,3H-呋喃并(3,4-c)呋喃-3-酮
去甲斑蝥素
单硝酸异山梨酯杂质C
单-9-十八烯酸1,4:3,6-双脱水-D-甘露醇酯
华北白前甙元B
六氢呋喃并[2,3-b]呋喃-3-醇
六氢呋喃并[2,3-b]呋喃
六氢-呋喃并[2,3-b]呋喃-3-醇
克罗拉滨杂质7
二氯萘
二氢-1,4-二甲基-1,4-乙桥-1H,3H-呋喃并(3,4-c)呋喃-3,6(4H)-二酮
二氢-1,4-乙桥-1H,3H-呋喃并(3,4-c)呋喃-3,6(4H)-二酮
二氢-1,4-乙桥-1H,3H-呋喃并(3,4-c)呋喃-3,6(4H)-二硫酮
乙酸异山梨醇酯
丙氨酸,N-(5-氯-2-羟基苯甲酰)-
N-乙酰基-L-丙氨酰-L-酪氨酸
L-葡糖酸-3,6-内酯
D-葡糖醛酸-γ-内酯丙酮化合物
D-甘露呋喃糖醛酸 gamma-内酯
BISTHFHNS衍生物3
7H,10H-呋喃并[2,3,4-cd]萘并[2,1-e]异苯并呋喃-7-酮,十四氢-10-羟基-1,1,4a-三甲基-,(4aS,4bR,6aR,8aR,10R,10aS,10bR,12aS)-(9CI)
7-氧杂二环[2.2.1]庚-5-烯-2,3-二羧酸酐
6H,9H-苯并[e]呋喃并[2,3,4-cd]异苯并呋喃-6-酮,2,4,4a,5,7,8,10a,10b-八氢-5,5-二甲基-,(4aR,8aR,10aR,10bS)-(9CI)
6-[(1E,3E,5E)-6-[(1R,2R,3R,5R,7R,8R)-7-乙基-2,8-二羟基-1,8-二甲基L-4,6-二氧杂双环[3.3.0]辛-3-基]己-1,3,5-三烯基]-4-甲氧基-5-甲基-吡喃-2-酮
5-单硝酸异山梨酯
5-乙酸异山梨酯
5-乙酸异山梨酯
5-[(4,6-二氯-1,3,5-三嗪-2-基)氨基]-4-羟基-3-[(4-磺酸根-1-萘基)偶氮]萘-2,7-二磺化三钠
5,6-二溴-7-氧杂双环[2.2.1]庚烷-2,3-二甲酸酐
5,5-二甲基-4,8-二氧杂三环[4.2.1.03,7]壬-2-基丙烯酸酯
4-硝基苯并[pqr]四苯-1-醇
4,10-二氧杂三环[5.2.1.0(2,6)]癸-8-烯-3-酮
4,10-二氧杂三环[5.2.1.0(2,6)]癸-8-烯-3,5-二酮
3-脱氧-14,15-二氢-15-羟基-莸酯素醇