1-甲基-4-亚甲基环己烷 | 2808-80-2
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:141.55°C (rough estimate)
-
密度:0.7910
-
保留指数:803.5
计算性质
-
辛醇/水分配系数(LogP):2.9
-
重原子数:8
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— (4-Methylenecyclohexylmethyl)bromide 76825-09-7 C8H13Br 189.095 4-亚甲基环己基甲醇 4-methylene-1-cyclohexanemethanol 1004-24-6 C8H14O 126.199
反应信息
-
作为反应物:描述:参考文献:名称:Thermolysis of alkyldioxetanes: effect of 3,3-cyclic substituents and conformation on the activation parameters摘要:DOI:10.1021/jo00197a034
-
作为产物:参考文献:名称:Wallach, Justus Liebigs Annalen der Chemie, 1909, vol. 365, p. 248摘要:DOI:
文献信息
-
Halogenated Terpenoids. XXXI. Tribromides from the Bromination of Various Exocyclic Olefins作者:Raymond M. Carman、Karl A. Hansford、Colin H. L. KennardDOI:10.1071/ch00041日期:——
Bromination of methylene groups exocyclic to cyclohexyl systems can afford, besides the expected trans-dibromo products, considerable quantities of a tribromide. For example, simple bromination of 4-t-butyl-1-methylidene-cyclohexane affords c. 20% yield of (r-1, t-2, c-4)-1,2-dibromo-1-bromomethyl-4-t-butylcyclohexane.
-
Stereochemistry and mechanism of catalytic hydrogenation of substituted methylenecyclohexanes作者:S. Mitsui、K. Gohke、H. Saito、A. Nanbu、Y. SendaDOI:10.1016/s0040-4020(01)83393-7日期:1973.1products were almost unity over freshly prepared Raney Ni but the axial Me counterparts were favoured over aged catalyst. The axial Me products were also preferred on Pt or Rh catalysts. Pd catalysed hydrogenation gave predominantly the equatorial Me isomers at high catalyst ratio, while the axial Me counterparts were favoured at the early stage of the reaction at a catalyst-substrate ratio of 1:20. The change
-
Construction of Distant Stereocenters by Enantioselective Desymmetrizing Carbonyl–Ene Reaction作者:Weiwei Luo、Lili Lin、Yu Zhang、Xiaohua Liu、Xiaoming FengDOI:10.1021/acs.orglett.7b01329日期:2017.7.7An efficient desymmetrizing carbonyl–ene reaction of 1-substituted 4-methylenecyclohexanes with glyoxal derivatives was thus executed by a chiral N,N′-dioxide/NiII catalyst, providing facile access to cyclohexene derivatives bearing two remote 1,6-related stereocenters. This distal stereocontrol methodology originates from the efficient interaction between the catalyst with enophiles, discrimination
-
Halogenated Terpenoids. XXIX The 1-Bromo 1-Bromomethyl Cyclohexyl System作者:Douglas J. Brecknell、Raymond M. Carman、Ross A. Edwards、Karl A. Hansford、Tomislav Karoli、Ward T. RobinsonDOI:10.1071/c96188日期:——
Bromination of methylene groups exocyclic to cyclohexyl systems normally affords two isomeric products; the axial 1-bromo equatorial 1-bromomethyl compound and the axial 1-bromomethyl equatorial 1-bromo derivative. Free energy differences between these two isomers, and the conformations adopted by the axial 1-bromomethyl group, have been explored by n.m.r. spectroscopy, by X-ray crystallography and by MM3 calculations. Evidence is presented to show that the ax-bromomethyl group exists primarily as those rotamers which site the bromine atom synclinal to the vicinal bromine. The A value for a bromomethyl group in this system is then similar to that of an unsubstituted methyl group.
-
Vinyllithium cyclizations with unactivated alkenes as internal electrophiles: stereoselective formation of substituted methylenecyclopentanes作者:A. Richard. Chamberlin、Steven H. Bloom、Laura A. Cervini、Christopher H. FotschDOI:10.1021/ja00222a042日期:1988.7Les vinyllithiums derives des triisopropyl-2,4,6 benzenesulfonylhydrazones correspondantes subissent une addition intramoleculaire pour donner des composes cycliques lithies qui peuvent etre pieges par des electrophiles tels que l'eau
表征谱图
-
氢谱1HNMR
-
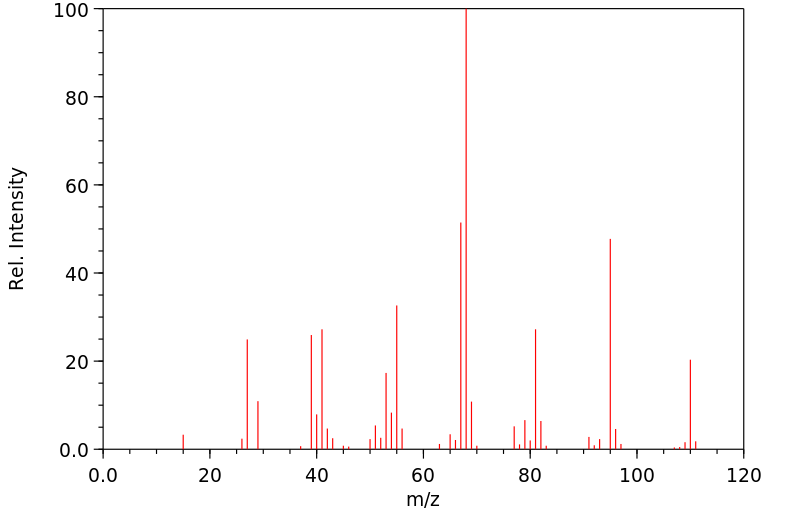
质谱MS
-
碳谱13CNMR
-
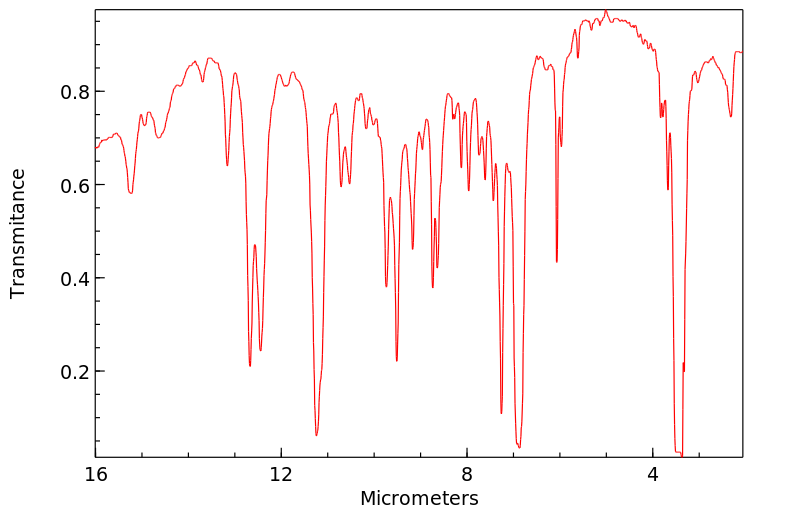
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息