dimethyl chelidonate | 6558-96-9
中文名称
——
中文别名
——
英文名称
dimethyl chelidonate
英文别名
dimethyl 4-oxo-4H-pyran-2,6-dicarboxylate;2,6-Dicarbomethoxy-4-pyron;Dimethyl-4-oxo-4H-pyran-2,6-dicarboxylat;Chelidonsaeure-dimethylester;4-oxo-4H-pyran-2,6-dicarboxylic acid dimethyl ester;4-Oxo-4H-pyran-2,6-dicarbonsaeure-dimethylester;2,6-dicarbomethoxy-4-pyrone;dimethyl 4-oxopyran-2,6-dicarboxylate
CAS
6558-96-9
化学式
C9H8O6
mdl
——
分子量
212.159
InChiKey
BHSUHVSNJNGPPV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):0.3
-
重原子数:15
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.22
-
拓扑面积:78.9
-
氢给体数:0
-
氢受体数:6
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— monomethyl chelidonate 6559-33-7 C8H6O6 198.132 4-氧代吡喃-2,6-二羧酸二乙基酯 diethyl 4-oxo-4H-pyran-2,6-dicarboxylate 725-92-8 C11H12O6 240.213 白屈菜酸 chelidonic acid 99-32-1 C7H4O6 184.105
反应信息
-
作为反应物:参考文献:名称:Attenburrow et al., Journal of the Chemical Society, 1945, p. 571,574摘要:DOI:
-
作为产物:参考文献:名称:New series of É£-pyrone based Podands: synthesis, characterization and study of their application in acetate salts cation trapping in nucleophilic substituted reactions摘要:Dialkyl 4-oxo-4H-pyran-2,6-dicarboxylates are synthesized via esterification of chelidonic acid or via intramolecular cyclization of dialkyl-2,4,6-trioxoheptanedioates. Reaction of the dialkyl 4-oxo-4H-pyran-2,6-dicarboxylates with a variety of glycol monoalkyl ethers produces a series of new podands in good yields. To demonstrate the use of these podands in cation trapping, nucleophilic substitution reactions are carried out with various acetate salts. The results indicate that the cation diameter's compatibility with binding site leads to the best yield of reaction.[GRAPHICS].DOI:10.24820/ark.5550190.p010.827
文献信息
-
CHARGE CONTROL AGENT AND RELATED ART申请人:Kuroda Kazuyoshi公开号:US20090053641A1公开(公告)日:2009-02-26[PROBLEMS] Providing a charge control agent that has a negative charge providing property at a practical level, is colorless to light-colored and usable in color toners, produces static charges stable to environmental changes by electrifying the resin powder of a toner and the like, is excellent in storage stability and durability, and is highly safe, as well as a method of controlling the charge of resin powder using the charge control agent, and a toner. [MEANS OF SOLVING THE PROBLEMS] A charge control agent having a compound represented by the formula shown below as the active ingredient, as well as a method of controlling the charge of resin powder using the charge control agent, and a toner. X: an oxygen atom or N—H; Each of R 1 to R 4 : a hydrogen atom, a carboxyl group, an aminocarbonyl group having or not having a substituent, an aminocarbonylmethyl group having or not having a substituent, an alkoxycarbonyl group, an alkyl group, a phenyl group having a substituent or not having a substituent, or a group that forms a saturated or unsaturated ring having or not having a substituent in cooperation with any other group selected from among R 1 to R 4 .
-
Ashok, D.; Sarma, P. N., Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 1988, vol. 27, # 9, p. 862作者:Ashok, D.、Sarma, P. N.DOI:——日期:——
-
KAPPLER D.; ROSENMUND P., CHEM. BER. <CHBE-AM>, 1976, 109, NO 10, 3486-3488作者:KAPPLER D.、 ROSENMUND P.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
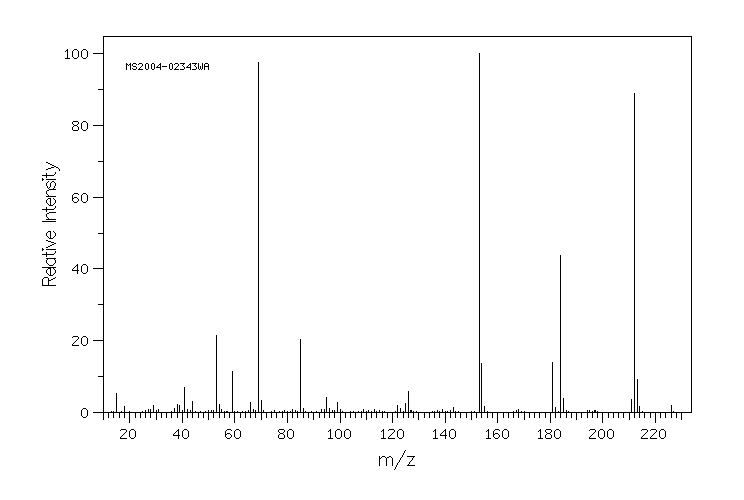
质谱MS
-
碳谱13CNMR
-
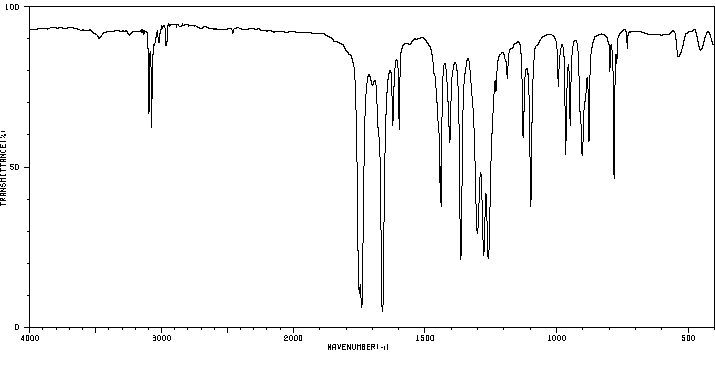
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2R)-2,6-二羟基-5-[(E)-丙-1-烯基]-1,2-二氢吡喃并[3,2-b]吡咯-3,7-二酮
黄绿青霉素
麦芽醇
麦芽酚铁
马索亚内酯
香豆酸
香豆灵酸甲酯
香叶吡喃
顺式-1-(3-呋喃基)-1,7,8,8a-四氢-5,8a-二甲基-3H-2-苯并吡喃-3-酮
靠曼酸乙酯; 4-吡喃酮-2-羧酸乙酯
靠曼酸
镭杂9蛋白质
铝3-羟基-2-甲基-4-吡喃酮
钠[(1E,7E,9E,11E)-6-羟基-1-(3-羟基-6-氧代-2,3-二氢吡喃-2-基)-5-甲基十七碳-1,7,9,11-四烯-4-基]硫酸盐
避虫酮
辛伐他汀杂质C
褐鸡蛋花素
脱氢乙酸缩氨基硫脲
脱氢乙酸
罌粟酸
维达列汀
福司曲星
福司曲星
磷内酯霉素F
磷内酯霉素E
磷内酯霉素D
磷内酯霉素A
白屈菜酸
甲基6-甲氧基-2-甲基-5-氧代四氢-2H-吡喃-2-羧酸酯
甲基6-氧杂双环[3.1.0]己烷-1-羧酸酯
甲基4-氧代-4H-吡喃-3-羧酸酯
甲基4,6-二-O-乙酰基-2,3-二脱氧己-2-烯基吡喃糖苷
甲基2H-吡喃-5-羧酸酯
甲基2-乙氧基-6-甲基-3,4-二氢-2H-吡喃-4-羧酸酯
甲基2-乙氧基-4-氧代-3,4-二氢-2H-吡喃-5-羧酸酯
甲基2-乙氧基-3-甲基-4-氧代-3,4-二氢-2H-吡喃-5-羧酸酯
甲基(4S)-2-氧代-4-[(2E)-1-氧代-2-丁烯-2-基]-3,4-二氢-2H-吡喃-5-羧酸酯
甲基(2S,5R)-5-甲氧基-3-硝基-2,5-二氢-2-呋喃羧酸酯
甲基(2S)-4-甲基-3,6-二氢-2H-吡喃-2-羧酸酯
甲基(2R)-四氢-2H-吡喃-2-羧酸酯
环庚三烯并[b]吡喃-2(5H)-酮,9-(3-丁烯基)-3-(环丙基苯基甲基)-6,7,8,9-四氢-4-羟基-
环吡酮杂质B
焦袂康酸O-甲基醚
沉香四醇
氨甲酸,[3-[(苯基甲基)氨基]三环[3.3.1.13,7]癸-1-基]-,1,1-二甲基乙基酯(9CI)
毛子草酮
棒曲霉素-13C3
棒曲霉素
木菌素
木糖酸二钠盐