3-O-(β-D-glucopyranosyl)-D-glucitol | 499-16-1
中文名称
——
中文别名
——
英文名称
3-O-(β-D-glucopyranosyl)-D-glucitol
英文别名
3-β-D-glucopyranosyl-D-glucitol;laminaribiitol;Lam-Glc2-ol;(2R,3R,4R,5S)-4-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyhexane-1,2,3,5,6-pentol
CAS
499-16-1
化学式
C12H24O11
mdl
——
分子量
344.316
InChiKey
VQHSOMBJVWLPSR-CRLSIFLLSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:788.5±60.0 °C(Predicted)
-
密度:1.69±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):-5.2
-
重原子数:23
-
可旋转键数:8
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:201
-
氢给体数:9
-
氢受体数:11
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— laminaribiose —— C12H22O11 342.3 —— Cellobiose 13360-52-6 C12H22O11 342.3 昆布二糖 laminaribiose 34980-39-7 C12H22O11 342.3 昆布六糖 laminarihexaose 29842-30-6 C36H62O31 990.87 黑曲霉三糖 laminaritriose —— C18H32O16 504.442 —— laminaritetraose —— C24H42O21 666.585 —— reduced laminaraheptaose —— C42H72O36 1153.01 —— 1-O-(β-D-glucopyranosyl)-2,4-O-benzylidene-5,6-O-isopropylidene-D-glucitol 876062-91-8 C22H32O11 472.489
反应信息
-
作为反应物:描述:3-O-(β-D-glucopyranosyl)-D-glucitol 在 三氧化硫 N,N-二甲基甲酰胺络合物 、 sodium acetate 、 sodium hydroxide 作用下, 以 N,N-二甲基甲酰胺 、 甲醇 、 乙醇 、 水 为溶剂, 反应 1.0h, 以99%的产率得到1,2,4,5,6-penta-O-sulfato-3-O-(2,3,4,6-tetra-O-sulfato-β-D-glucopyranosyl)-D-glucitol nonasodium salt参考文献:名称:[EN] POLYSULFATED GLYCOSIDES AND SALTS THEREOF
[FR] GLYCOSIDES POLYSULFATES ET SELS DE CEUX-CI摘要:公开号:WO2006017726A3 -
作为产物:描述:laminaribiose 在 sodium tetrahydroborate 作用下, 生成 3-O-(β-D-glucopyranosyl)-D-glucitol参考文献:名称:Co-ordinated synthesis of gentiobiitol and sorbitol, evidence of sorbitol glycosylation in transgenic sugarcane摘要:Sugarcane (a Saccharum spp. interspecific hybrid) was previously engineered to synthesize sorbitol (designated as sorbitolcane). Motivated by the atypical development of the leaves in some sorbitolcane, the polar metabolite profiles in the leaves of those plants were compared against a group of control sugarcane plants. Eighty-six polar metabolites were detected in leaf extracts by GC-MS. Principal component analysis of the metabolites indicated that three compounds were strongly associated with sorbitolcane. Two were identified as sorbitol and gentiobiose and the third was unknown. Gentiobiose and the unknown compound were positively correlated with sorbitol accumulation. The unknown compound was only abundant in sorbitolcane. This compound was structurally characterized and found to be a sorbitol-glucose conjugate. C-13 NMR analysis indicated that the glucopyranose and glucitol moieties were 1,6-linked. Ligand exchange chromatography confirmed that the compound was a beta-anomer, thus identifying the compound as 6-O-beta-D-glucopyranosyl-D-glucitol, or gentiobiitol. (C) 2010 Elsevier Ltd. All rights reserved.DOI:10.1016/j.phytochem.2010.01.014
文献信息
-
Exploring novel non-Leloir β-glucosyltransferases from proteobacteria for modifying linear (β1→3)-linked gluco-oligosaccharide chains作者:Gudmundur O Hreggvidsson、Justyna M Dobruchowska、Olafur H Fridjonsson、Jon O Jonsson、Gerrit J Gerwig、Arnthor Aevarsson、Jakob K Kristjansson、Delphine Curti、Robert R Redgwell、Carl-Eric Hansen、Johannis P Kamerling、Takoua Debeche-BoukhitDOI:10.1093/glycob/cwq165日期:2011.3Over the years several β-glucan transferases from yeast and fungi have been reported, but enzymes with such an activity from bacteria have not been characterized so far. In this work, we describe the cloning and expression of genes encoding β-glucosyltransferase domains of glycosyl hydrolase family GH17 from three species of proteobacteria: Pseudomonas aeruginosa PAO1, P. putida KT2440 and Azotobacter vinelandii ATCC BAA-1303. The encoded enzymes of these GH17 domains turned out to have a non-Leloir trans-β-glucosylation activity, as they do not use activated nucleotide sugar as donor, but transfer a glycosyl group from a β-glucan donor to a β-glucan acceptor. More particularly, the activity of the three recombinant enzymes on linear (β1 → 3)-linked gluco-oligosaccharides (Lam-Glc4–9) and their corresponding alditols (Lam-Glc4–9-ol) was studied. Detailed structural analysis, based on thin-layer chromatography, matrix-assisted laser desorption ionization time-of-flight mass spectrometry, electrospray ionization mass spectrometry, and 1D/2D 1H and 13C nuclear magnetic resonance data, revealed diverse product spectra. Depending on the enzyme used, besides (β1 → 3)-elongation activity, (β1 → 4)- or (β1 → 6)-elongation, or (β1 → 6)-branching activities were also detected.多年来,酵母和真菌中的β-葡聚糖转移酶已被报道,但细菌中具有这种活性的酶迄今尚未被鉴定。在这项工作中,我们描述了三种蛋白细菌中编码糖基水解酶家族 GH17 β-葡聚糖转移酶结构域的基因的克隆和表达:铜绿假单胞菌(Pseudomonas aeruginosa PAO1)、腐生假单胞菌(P. putida KT2440)和乙烯绿氮菌(AzotoBActer vinelandii ATCC BAA-1303)。这些 GH17 结构域编码的酶具有非 Leloir 反式-β-葡萄糖基化活性,因为它们不使用活化的核苷酸糖作为供体,而是将一个糖基从β-葡聚糖供体转移到β-葡聚糖受体。更具体地说,研究了这三种重组酶对线性(β1 → 3)连接的葡聚寡糖(Lam-Glc4-9)及其相应的醛醇(Lam-Glc4-9-ol)的活性。基于薄层色谱法、基质辅助激光解吸电离飞行时间质谱法、电喷雾电离质谱法以及 1D/2D 1H 和 13C 核磁共振数据的详细结构分析揭示了不同的产物光谱。根据所用酶的不同,除了(β1 → 3)-伸长活性外,还检测到(β1 → 4)-或(β1 → 6)-伸长或(β1 → 6)-分支活性。
-
POLYSULFATED GLYCOSIDES AND SALTS THEREOF申请人:Kuszmann János公开号:US20110118198A1公开(公告)日:2011-05-19The invention relates to polysulfated glycosides of formula (I), the pharmaceutically acceptable salts thereof, as well as the pharmaceutical compositions containing these compounds as active ingredients. Furthermore the invention provides a method of preventing, treating or alleviating the symptoms of acute and chronic inflammatory disorders of the airways of mammals—including asthma and asthma-related pathologies.本发明涉及公式(I)的多硫酸化糖苷,其药学上可接受的盐,以及包含这些化合物作为活性成分的制药组合物。此外,本发明提供了一种预防、治疗或缓解哺乳动物呼吸道急性和慢性炎症性疾病症状的方法,包括哮喘和哮喘相关病理。
-
Two approaches to the synthesis of 3-β-d-glucopyranosyl-d-glucitol作者:János Kuszmann、Gábor Medgyes、Sándor BorosDOI:10.1016/j.carres.2004.07.019日期:2004.10Glycosidation of 1,2:5,6-di-O-isopropylidene-D-glucose with tetra-O-acetyl-glucosyl bromide in 1:1 benzene-MeNO2 afforded approximately equal amounts of the 3-O-beta-D-glycoside and the rearranged 6-O-beta-D-glycoside, while in MeCN only the latter was formed. When tetra-O-acetyl-beta-thiophenylgiucoside was used as donor in CH2Cl2 in the presence of NIS/TfOH as activator, the 6-O-beta-D-glycoside and a 3-O-orthoester were formed in a 1:2 ratio at -20 degreesC, while at 20 degreesC only the former could be isolated. Glycosidation of 1-O-benzoyl-2,4-0-benzylidene-5,6-O-isopropylidene-D-glycutol with tetra-O-acetyl-glucosyl bromide in MeCN in the presence of Hg(CN)(2) afforded the corresponding 3-O-alpha- and 3-O-beta-glycopyranoside in a 1:4 ratio in MeCN and 1:5 in 1:1 benzene-MeNO2. respectively. When Hg(CN)(2)/HgBr2 was used as promoter, the corresponding orthoester was also formed. When tetra-O-acetyl-beta-thiophenylglucoside was used as donor, the 3-O-beta-anomer and the orthoester were obtained predominantly in a 3:2 ratio together with traces of the 3-O-alpha-glycoside. Both beta-glycosides could be smoothly converted into 3-beta-D-glucopyranosyl-D-glucitol (C) 2004 Elsevier Ltd. All rights reserved.
-
One-Step Conversion of Cellobiose to C<sub>6</sub>-Alcohols Using a Ruthenium Nanocluster Catalyst作者:Ning Yan、Chen Zhao、Chen Luo、Paul J. Dyson、Haichao Liu、Yuan KouDOI:10.1021/ja062468t日期:2006.7.1The one-step conversion of cellulose to C6-alcohols via green and energy efficient approaches has, as far as we are aware, not been reported. Such a process presents a considerable challenge, the two key problems being (1) finding a suitable solvent that dissolves the cellulose, and (2) the development of advanced catalytic chemistry for selective cleavage of the C-O-C bonds (glycosidic bonds) connecting glucose residues. The dissolution of cellulose has been recently realized by using ionic liquids as green solvents; there is still no efficient method, such as selective hydrogenation, for the precise C-O-C cleavage under mild conditions, however. Cellobiose is a glucose dimer connected by a glycosidic bond and represents the simplest model molecule for cellulose. We disclose in this communication that the one-step conversion of cellobiose to C6-alcohols can be realized by selectively breaking the C-O-C bonds via selective hydrogenation using a water-soluble ruthenium nanocluster catalyst under 40 bar H2 pressure.
-
SEQUENTIAL REMOVAL OF MONOSACCHARIDES FROM THE REDUCING END OF OLIGOSACCHARIDES AND USES THEREOF申请人:The Biomembrane Institute公开号:EP0649427A1公开(公告)日:1995-04-26
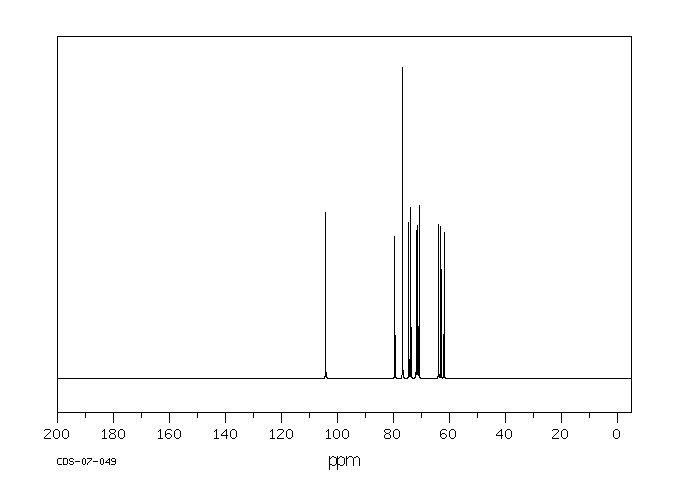
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯