5-methyl-5-(p-tolyl)dihydrofuran-2(3H)-one | 21034-34-4
中文名称
——
中文别名
——
英文名称
5-methyl-5-(p-tolyl)dihydrofuran-2(3H)-one
英文别名
γ-p-Tolyl-γ-valerolacton;5-Methyl-5-(4-methylphenyl)oxolan-2-one
CAS
21034-34-4
化学式
C12H14O2
mdl
——
分子量
190.242
InChiKey
SXSKLDSQMFZPAR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:51.3-52 °C
-
沸点:167-168 °C(Press: 8 Torr)
-
密度:1.086±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:14
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.42
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
反应信息
-
作为反应物:描述:5-methyl-5-(p-tolyl)dihydrofuran-2(3H)-one 在 palladium 10% on activated carbon 、 氢气 、 sodium acetate 、 三溴化磷 作用下, 以 氯仿 、 乙酸乙酯 为溶剂, 反应 26.17h, 生成 1-bromo-4,7-dimethyl-3,4-dihydro-naphthalene-2-carbaldehyde参考文献:名称:杂原子导向的Wacker氧化。( - ) -的无保护合成heliophenanthrone † ‡摘要:第一对映体的六步合成 (-)-螺菲蒽酮无需任何保护-脱保护方案即可实现,总收率为28%。该合成的关键特征包括除不对称布朗烯丙基化和闭环复分解(RCM)之外,内部环烯烃的杂原子定向瓦克氧化。DOI:10.1039/c2ob07095b
-
作为产物:描述:3-(4-甲基苯甲酰)丙酸 在 六氟异丙醇 、 potassium tert-butylate 、 rhenium(VII) oxide 作用下, 以 四氢呋喃 为溶剂, 反应 17.5h, 生成 5-methyl-5-(p-tolyl)dihydrofuran-2(3H)-one参考文献:名称:Re2O7/HReO4 介导的未活化烯烃的分子内加氢酰氧基化:双重氢键效应摘要:该出版物描述了 Re 2 O 7在六氟异丙醇 (HFIP) 中的应用,用于活化惰性和电子失活的烯烃,以促进具有挑战性的分子内加氢酰氧基化反应。HFIP 和内部羧基均已被证明对于成功实施这一转变至关重要;这些被提议通过与高铼酸盐阴离子 (ReO 4 – )的氢键相互作用来帮助关键阳离子中间体的形成和稳定。DOI:10.1021/acs.orglett.2c03846
文献信息
-
CHEMOSPECIFICITY IN ARYLATIONS OF δ- AND γ-KETOCARBOXYLIC ACIDS WITH P<sub>2</sub>O<sub>5</sub>–MsOH, TfOH, AND RELATED ACIDIC MEDIA作者:Noriyuki Yonezawa、Masayuki Koike、Asami Kameda、Shin Naito、Tetsuo Hino、Katsuya Maeyama、Tomiki IkedaDOI:10.1081/scc-120013730日期:2002.1.1Remarkable contrast between chemospecificities in acid-mediated arylation of δ- and γ-ketocarboxylic acids was revealed: in the presence of P2O5–MsOH, TfOH, PPA, and MsOH, arylation of δ-ketocarboxylic acid 1A with arenes takes place at the carboxycarbonyl carbon, while that of γ-ketocarboxylic acid 1B takes place at the ketone carbonyl carbon, specifically.
-
Copper‐Catalyzed Formal [2+2+1] Heteroannulation of Alkenes, Alkylnitriles, and Water: Method Development and Application to a Total Synthesis of (±)‐Sacidumlignan D作者:Tu M. Ha、Claire Chatalova‐Sazepin、Qian Wang、Jieping ZhuDOI:10.1002/anie.201604528日期:2016.8A copper‐catalyzed three‐component reaction of alkenes, alkylnitriles, and water affords γ‐butyrolactones in good yields. The domino process involves an unprecedented hydroxy‐cyanoalkylation of alkenes and subsequent lactonization with the creation of three chemical bonds and a quaternary carbon center. The synthetic potential of this novel [2+2+1] heteroannulation reaction was illustrated by a concise
-
ZnI<sub>2</sub>/Zn(OTf)<sub>2</sub>-TsOH: a versatile combined-acid system for catalytic intramolecular hydrofunctionalization and polyene cyclization作者:Ting-Hung Chou、Bo-Hung Yu、Rong-Jie CheinDOI:10.1039/c9cc07242j日期:——combined-acid system using a zinc(II) salt [ZnI2 or Zn(OTf)2] and p-toluene sulfonic acid (TsOH) was investigated for catalytic cationic cyclizations, including intramolecular hydrocarboxylation, hydroalkoxylation, hydroamination, hydroamidation, hydroarylation and polyene cyclizations. This reaction provides easy access to five- and six-membered O- and N-containing saturated heterocyclic compounds, tetrahydronaphthalene
-
Electron-transfer processes作者:Claudio Giordano、Aldo Belli、Attilio Citterio、Francesco MinisciDOI:10.1016/0040-4020(80)88052-5日期:1980.1A new synthesis of γ-lactones by peroxydisulfate oxidation of isopropylbenzenes is described. Evidence concerning the free-radical mechanism is reported; the intermediate formation of alkyl and benzyl radicals is evidenced by trapping them with quinoxaline. The role of the iron catalyst is discussed.
-
Divergent functionalization of alkenes enabled by photoredox activation of CDFA and α-halo carboxylic acids作者:Rahul Giri、Egor Zhilin、Dmitry KatayevDOI:10.1039/d4sc01084a日期:——present our studies on the solvent-controlled difunctionalization of alkenes utilizing chlorodifluoroacetic acid (CDFA) and α-halo carboxylic acids for the synthesis of γ-lactones, γ-lactams and α,α-difluoroesters. Mechanistic insights revealed that photocatalytic reductive mesolytic cleavage of the C–X bond delivers elusive α-carboxyl alkyl radicals. In the presence of an olefin molecule, this species在此,我们介绍了利用氯二氟乙酸 (CDFA) 和 α-卤代羧酸对烯烃进行溶剂控制双官能化以合成 γ-内酯、γ-内酰胺和 α,α-二氟酯的研究。机理研究表明,C-X 键的光催化还原介观裂解会产生难以捉摸的 α-羧基烷基自由基。在烯烃分子存在的情况下,该物质充当独特的双功能中间体,允许通过单电子转移 (SET) 或氢原子转移在吉斯型加合物上形成规定的 C-O、C-N 和 C-H 键( HAT)事件。这些方案在多种容易获得的 α-卤代羧酸中表现出极高的效率,并且适合批量和流动的可扩展性。为了证明这一概念的多功能性,成功合成了 (±)-boivinianin A、其氟化类似物和 eupomatilone-6 天然产物。
表征谱图
-
氢谱1HNMR
-
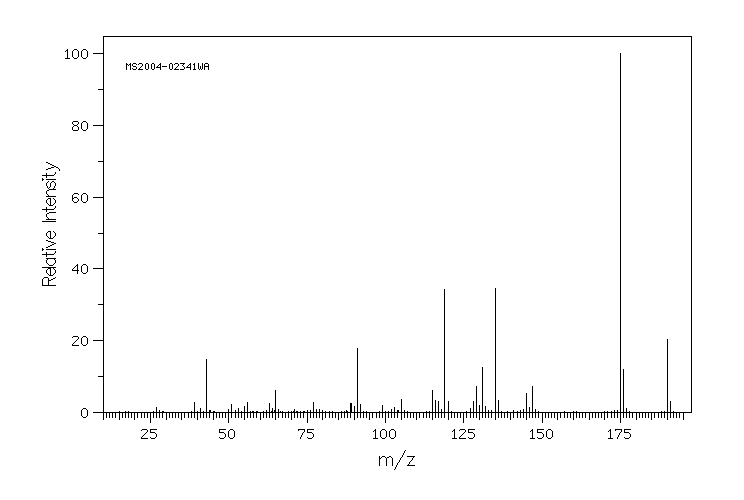
质谱MS
-
碳谱13CNMR
-
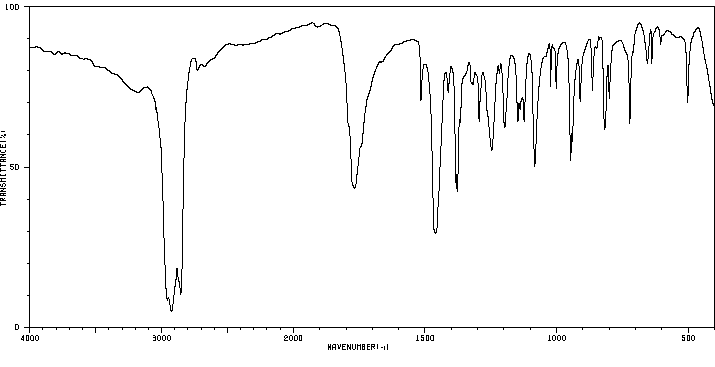
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫