2-(3-氯苯氧基)硫代乙酰胺 | 35370-95-7
中文名称
2-(3-氯苯氧基)硫代乙酰胺
中文别名
2-(3-氯苯氧基)乙烷硫代酰胺
英文名称
2-(3-chlorophenoxy) ethanethioamide
英文别名
2-(3-chlorophenoxy)ethanethioamide;MBF2;2-(3-chloro-phenoxy)-thioacetamide;(3-Chlor-phenoxy)-thioessigsaeure-amid
CAS
35370-95-7
化学式
C8H8ClNOS
mdl
——
分子量
201.677
InChiKey
RPAOLVIADVQKNA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:122-124
-
沸点:338.6±48.0 °C(Predicted)
-
密度:1.339±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:12
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:67.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
海关编码:2930909090
SDS
| Name: | 2-(3-Chlorophenoxy)ethanethioamide 95+% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 35370-95-7 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 35370-95-7 | 2-(3-Chlorophenoxy)ethanethioamide | 95+% | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 35370-95-7: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white crystalline solid
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 122 - 124 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H8ClNOS
Molecular Weight: 202
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Oxidizing agents, bases, amines.
Hazardous Decomposition Products:
Hydrogen chloride, chlorine, nitrogen oxides, carbon monoxide, oxides of sulfur, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 35370-95-7 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2-(3-Chlorophenoxy)ethanethioamide - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 35370-95-7: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 35370-95-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 35370-95-7 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-(3-氯苯氧基)乙酰胺 2-(3-chlorophenoxy)acetamide 35368-69-5 C8H8ClNO2 185.61 3-氯苯氧乙腈 (3-chloro-phenoxy)-acetonitrile 43111-32-6 C8H6ClNO 167.595 间氯苯氧乙酸 3-chlorophenoxyacetic acid 588-32-9 C8H7ClO3 186.595 3-氯苯氧基乙酰基氯 2-(3-chlorophenoxy)acetyl chloride 114476-84-5 C8H6Cl2O2 205.04 2-(3-氯苯氧基)乙酸甲酯 methyl m-chlorophenoxyacetate 74411-14-6 C9H9ClO3 200.622
反应信息
-
作为反应物:描述:2-(3-氯苯氧基)硫代乙酰胺 在 aluminum (III) chloride 作用下, 以 甲醇 、 二硫化碳 为溶剂, 反应 28.5h, 生成 2-[3-chloro-4-(1-oxopropyl)phenoxymethyl]-4-(4-trifluoromethylphenyl)thiazole参考文献:名称:乙二酸噻唑衍生物作为谷胱甘肽S-转移酶pi抑制剂的合成摘要:谷胱甘肽S-转移酶pi(GSTpi)是II期酶,可保护细胞免于死亡,并清除癌细胞中的化学治疗剂。乙炔酸(EA)是一种弱的GSTpi抑制剂。进行结构修饰以提高EA抑制GSTpi活性的能力。设计并合成了18种EA噻唑衍生物。用杂环噻唑取代EA的羧基的化合物9a,9b和9c显示出相对于EA的改善,以抑制GSTpi活性。DOI:10.1016/j.bmc.2012.02.011
-
作为产物:描述:间氯苯氧乙酸 在 氯化亚砜 、 tetraphosphorus decasulfide 、 氨 作用下, 以 四氢呋喃 、 水 、 甲苯 为溶剂, 反应 7.0h, 生成 2-(3-氯苯氧基)硫代乙酰胺参考文献:名称:乙二酸噻唑衍生物作为谷胱甘肽S-转移酶pi抑制剂的合成摘要:谷胱甘肽S-转移酶pi(GSTpi)是II期酶,可保护细胞免于死亡,并清除癌细胞中的化学治疗剂。乙炔酸(EA)是一种弱的GSTpi抑制剂。进行结构修饰以提高EA抑制GSTpi活性的能力。设计并合成了18种EA噻唑衍生物。用杂环噻唑取代EA的羧基的化合物9a,9b和9c显示出相对于EA的改善,以抑制GSTpi活性。DOI:10.1016/j.bmc.2012.02.011
文献信息
-
[EN] SUBSTITUTED THIAZOLES FOR THE TREATMENT OF INFLAMMATION<br/>[FR] THIAZOLES SUBSTITUES DESTINES AU TRAITEMENT DE L'INFLAMMATION申请人:G.D. SEARLE & CO.公开号:WO1996003392A1公开(公告)日:1996-02-08(EN) A class of substituted thiazolyl compounds is described for use in treating inflammation and inflammation-related disorders. Compounds of particular interest are defined by Formula (II), wherein R4 is selected from alkyl and amino, wherein R5 is selected from aryl, cycloalkyl, cycloalkenyl and heterocyclic; wherein R5 is optionally substituted at a substitutable position with one or more radicals selected from halo, alkylthio, alkylsulfinyl, alkylsulfonyl, haloalkylsulfonyl, aminosulfonyl, alkyl, alkenyl, alkynyl, cyano, carboxyl, carboxyalkyl, alkoxycarbonyl, aminocarbonyl, acyl, N-alkylaminocarbonyl, N-arylaminocarbonyl, N,N-dialkylaminocarbonyl, N-alkyl-N-arylaminocarbonyl, haloalkyl, hydroxyl, alkoxy, hydroxyalkyl, haloalkoxy, amino, N-alkylamino, N,N-dialkylamino, heterocyclic and nitro; and wherein R6 is selected from halo, amino, alkoxy, nitro, hydroxyl, aminocarbonyl, acyl, alkylaminocarbonyl, arylaminocarbonyl, alkenyl, alkynyl, haloalkoxy, alkylamino, arylamino, aralkylamino, alkoxycarbonylalkyl, alkylaminoalkyl, heterocycloalkyl, aralkyl, cyanoalkyl, N-alkylsulfonylamino, heteroarylsulfonylalkyl, heteroarylsulfonylhaloalkyl, aryloxyalkyl, aralkyloxyalkyl, aryl and heterocyclo, wherein the aryl and heterocyclo radicals are optionally substituted at a substitutable position with one or more radicals selected from halo, alkyl, alkoxy, alkylthio, alkylsulfinyl, haloalkyl, haloalkoxy, carboxyalkyl, alkoxycarbonyl, aminocarbonyl, amino, acyl and alkylamino; or a pharmaceutically-acceptable salt thereof.(FR) La présente invention concerne une classe de composés à base de thiazolyle substitué destinés au traitement de l'inflammation et des troubles liés à l'inflammation. Les composés concernés en l'occurrence sont décrits par la formule générale (II). Dans cette formule générale, R4 est choisi parmi alkyle et amino. R5 est choisi parmi aryle, cycloalkyle, cycloalcényle et hétérocyclique. R5 est éventuellement substitué à des positions admettant la substitution par un ou plusieurs radicaux choisis parmi halo, alkylthio, alkylsulfinyle, alkylsulfonyle, haloalkylsulfonyle, aminosulfonyle, alkyle, alcényle, alkynyle, cyano, carboxyle, carboxyalkyle, alcoxycarbonyle, aminocarbonyle, acyle, N-alkylaminocarbonyle, N-arylaminocarbonyle, N,N-dialkylaminocarbonyle, N-alkyl-N-alkylaminocarbonyle, haloalkyle, hydroxyle, alcoxy, hydroxyalkyle, haloalcoxy, amino, N-alkylamino, N,N-dialkylamino, hétérocyclique et nitro. Dans cette formule générale, R6 est choisi parmi halo, amino, alcoxy, nitro, hydroxyle, aminocarbonyle, acyle, alkylaminocarbonyle, arylaminocarbonyle, alcényle, alkynyle, haloalcoxy, alkylamino, arylamino, aralkylamino, alcoxycarbonylalkyle, alkylaminoalkyle, hétérocycloalkyle, aralkyle, cyanoalkyle, N-alkysulfonylamino, hétéroarylsulfonylalkyle, hétéroarylsulfonylhaloalkyle, aryloxyalkyle, aralkyloxyalkyle, aryle et hétérocyclo, où les radicaux aryle et hétérocyclo peuvent être éventuellement substitués à une position admettant la substitution par au moins un radical choisi parmi halo, alkyle, alcoxy, alkylthio, alkylsulfinyle, haloalkyle, haloalcoxy, carboxyalkyle, alcoxycarbonyle, aminocarbonyle, amino, acyle et alkylamino. L'invention concerne également des sels de ces composés de formule générale (II), qui sont pharmaceutiquement acceptables.描述了一类取代噻唑基化合物,用于治疗炎症和与炎症相关的疾病。特别感兴趣的化合物由公式(II)定义,其中R4选择自烷基和氨基,其中R5选择自芳基,环烷基,环烯基和杂环;其中R5在可取代的位置上可选择用一个或多个基团进行取代,所述基团选择自卤素,烷硫基,烷基亚砜基,烷基磺酰基,卤代烷基磺酰基,氨基磺酰基,烷基,烯基,炔基,氰基,羧基,羧基烷基,烷氧基羧酸酯基,氨基羧酸酯基,酰基,N-烷基氨基羧酸酯基,N-芳基氨基羧酸酯基,N,N-二烷基氨基羧酸酯基,N-烷基-N-芳基氨基羧酸酯基,卤代烷基,羟基,烷氧基,卤代烷氧基,氨基,N-烷基氨基,N,N-二烷基氨基,杂环和硝基;其中R6选择自卤素,氨基,烷氧基,硝基,羟基,氨基羧酸酯基,酰基,烷基氨基羧酸酯基,芳基氨基羧酸酯基,烯基,炔基,卤代烷氧基,烷基氨基,芳基氨基,芳基烷基氨基,烷氧基羧酸酯基烷基,烷基氨基烷基,杂环环烷基,芳基烷基,卤代基烷基磺酰氨基,杂环芳基磺酰基烷基,杂环芳基磺酰卤代烷基,芳氧基烷基,芳基烷氧基烷基,芳基和杂环,其中芳基和杂环基团在可取代的位置上可选择用一个或多个基团进行取代,所述基团选择自卤素,烷基,烷氧基,烷硫基,烷基亚砜基,卤代烷基,卤代烷氧基,羧基烷基,烷氧基羧酸酯基,氨基羧酸酯基,氨基,酰基和烷基氨基;或其药学上可接受的盐。
-
Substituted thiazoles for the treatment of inflammation申请人:G.D. Searle & Co.公开号:US05668161A1公开(公告)日:1997-09-16A class of substituted thiazolyl compounds is described for use in treating inflammation disorders. Compounds are defined by Formula II ##STR1## wherein R.sup.1 is selected from hydrido, alkyl, haloalkyl, cyanoalkyl, alkylamino, aralkyl, arylamino, heteroarylsulfonylalkyl, heteroarylsulfonylhaloalkyl, aralkylamino, aryloxyalkyl, alkoxycarbonyl, aryl optionally substituted at a substitutable position with one or more radicals selected from halo and alkoxy, and heterocyclic optionally substituted at a substitutable position with one or more radicals selected from halo and alkyl; wherein R.sup.4 is selected from alkyl and amino; and wherein R.sup.5 is selected from aryl and heteroaryl; wherein R.sup.5 is optionally substituted at a substitutable position with one or more radicals selected from halo, alkyl and alkoxy; provided R.sup.5 is not phenyl at position 4 when R.sup.1 is .alpha.,.alpha.-bis(trifluoromethyl)methanol and R.sup.4 is methyl; or a pharmaceutically-acceptable salt thereof.本文描述了一类取代噻唑基化合物,用于治疗炎症性疾病。化合物由式子II定义,其中R.sup.1选自氢,烷基,卤代烷基,氰基烷基,烷基氨基,芳基烷基,芳基氨基,杂环磺酰基烷基,杂环磺酰卤代烷基,芳基烷基氨基,芳氧基烷基,烷氧羰基,芳基(在可取代位置上可选地被一个或多个卤基和烷氧基基团取代)和杂环(在可取代位置上可选地被一个或多个卤基和烷基基团取代);其中R.sup.4选自烷基和氨基;其中R.sup.5选自芳基和杂环;其中R.sup.5在可取代位置上可选地被一个或多个卤基,烷基和烷氧基基团取代;但当R.sup.1为.alpha.,.alpha.-双三氟甲基甲醇,R.sup.4为甲基时,R.sup.5不得为苯基在4位上;或其药学上可接受的盐。
-
2-(ARYLOXYMETHYL)THIAZOLINES AND PENTHIAZOLINES作者:CARL DJERASSI、CAESAR R. SCHOLZDOI:10.1021/jo01149a041日期:1950.5
-
Abdel-Lateef,M.F.-A.; Eckstein,Z., Bulletin de l'Academie Polonaise des Sciences, Serie des Sciences Chimiques, 1971, vol. 19, # 11-12, p. 705 - 711作者:Abdel-Lateef,M.F.-A.、Eckstein,Z.DOI:——日期:——
-
Abdel-Lateef,M.F.-A. et al., Roczniki Chemii, 1976, vol. 50, p. 323 - 327作者:Abdel-Lateef,M.F.-A. et al.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
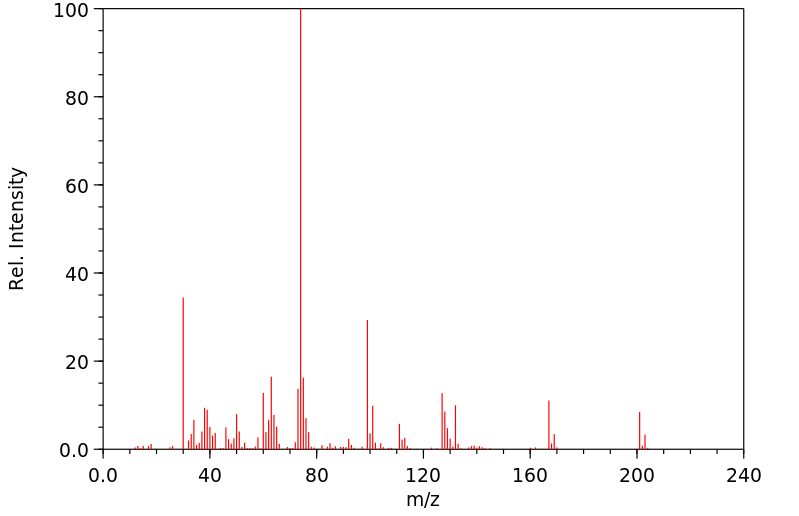
质谱MS
-
碳谱13CNMR
-
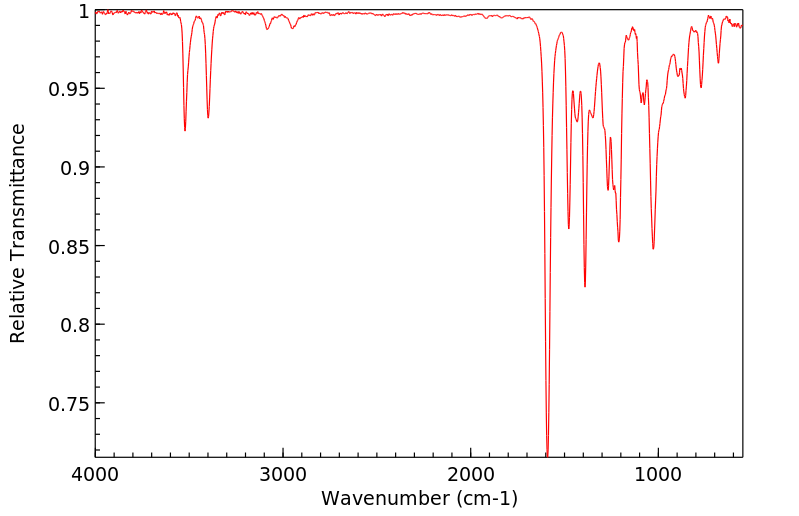
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯