(S)-(-)-乳酸异丙酯 | 63697-00-7
中文名称
(S)-(-)-乳酸异丙酯
中文别名
L-乳酸异丙脂
英文名称
(S)-isopropyl lactate
英文别名
isopropyl (S)-lactate;(S)-isopropyl 2-hydroxypropanoate;isopropyl lactate;isopropyl (S)-2-hydroxypropanoate;isopropyl L-lactate;Propanoic acid, 2-hydroxy-, 1-methylethyl ester, (2S)-;propan-2-yl (2S)-2-hydroxypropanoate
CAS
63697-00-7
化学式
C6H12O3
mdl
——
分子量
132.159
InChiKey
KIWATKANDHUUOB-YFKPBYRVSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:1 °C
-
沸点:166-168 °C(lit.)
-
密度:0.988 g/mL at 25 °C(lit.)
-
蒸气密度:4.2 (vs air)
-
闪点:135 °F
-
LogP:0.314 (est)
-
稳定性/保质期:
遵照规定使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:9
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.83
-
拓扑面积:46.5
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险等级:3.2
-
危险品标志:Xi
-
危险类别码:R66
-
危险品运输编号:UN 3272 3
-
WGK Germany:3
-
包装等级:III
-
危险类别:3.2
-
安全说明:S23,S24/25
-
储存条件:应存放在阴凉干燥的环境中。
SDS
| Name: | Isopropyl(S)-(-)-lactate 99% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 63697-00-7 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 63697-00-7 | Isopropyl(S)-(-)-lactate, 99% | 99 | 264-417-1 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Vapors may cause dizziness or suffocation.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Vapors may form an explosive mixture with air.
Vapors can travel to a source of ignition and flash back. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Will burn if involved in a fire. Use water spray to keep fire-exposed containers cool. Flammable liquid and vapor. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Containers may explode when heated.
Extinguishing Media:
For small fires, use dry chemical, carbon dioxide, water spray or alcohol-resistant foam. For large fires, use water spray, fog, or alcohol-resistant foam. Use water spray to cool fire-exposed containers. Use agent most appropriate to extinguish fire. Do NOT use straight streams of water.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Use a spark-proof tool. Provide ventilation. A vapor suppressing foam may be used to reduce vapors.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use only in a well-ventilated area.
Ground and bond containers when transferring material. Use spark-proof tools and explosion proof equipment. Avoid contact with eyes, skin, and clothing. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep container tightly closed. Keep away from heat, sparks and flame. Avoid ingestion and inhalation. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames.
Storage:
Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Flammables-area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 63697-00-7: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: mPas 20 deg C
Boiling Point: 166 - 168 deg C @ 760.00mm Hg
Freezing/Melting Point: 0.5 deg C
Autoignition Temperature: > 380 deg C (> 716.00 deg F)
Flash Point: 57 deg C ( 134.60 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: >157 deg C
Solubility in water: miscible with most organic solvents
Specific Gravity/Density: .9880g/cm3
Molecular Formula: C6H12O3
Molecular Weight: 132.16
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, ignition sources, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong reducing agents, strong acids, strong bases.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 63697-00-7 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Isopropyl(S)-(-)-lactate, 99% - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: FLAMMABLE LIQUID, N.O.S.*
Hazard Class: 3
UN Number: 1993
Packing Group: III
IMO
Shipping Name: FLAMMABLE LIQUID, N.O.S.
Hazard Class: 3.3
UN Number: 1993
Packing Group: III
RID/ADR
Shipping Name: FLAMMABLE LIQUID, N.O.S.
Hazard Class: 3
UN Number: 1993
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 9 Keep container in a well-ventilated place.
S 16 Keep away from sources of ignition - No
smoking.
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 33 Take precautionary measures against static
discharges.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 63697-00-7: No information available.
Canada
CAS# 63697-00-7 is listed on Canada's NDSL List.
CAS# 63697-00-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 63697-00-7 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 L-丙交酯 L,L-dilactide 4511-42-6 C6H8O4 144.127
反应信息
-
作为反应物:描述:(S)-(-)-乳酸异丙酯 在 dirhodium tetraacetate 、 二苯基磷酸 、 mercury(II) diacetate 、 三乙胺 、 三氟乙酸 作用下, 以 二氯甲烷 为溶剂, 反应 197.0h, 生成 isopropyl trans-(2S)-2-(2-[(tert-butoxycarbonyl)amino]cyclopropyloxy)propanoate参考文献:名称:Synthesis of cyclopropanoid 2-epi-muramyldipeptide analogues as potential immunostimulants摘要:The preparation of cyclopropanoid 2-epi-muramyldipeptide analogues from suitable substituted cyclopropylamines is described. (C) 2004 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tet.2004.01.037
-
作为产物:描述:参考文献:名称:[EN] ANTIVIRAL PRODRUGS OF TENOFOVIR
[FR] PROMÉDICAMENTS ANTIVIRAUX DU TÉNOFOVIR摘要:化合物I的公式及其药用盐和共晶体对HIV逆转录酶的抑制是有用的。这些化合物也可能对HIV感染的预防或治疗以及对AIDS的预防、延迟发病或进展和治疗具有用处。这些化合物及其盐可作为药物组合物中的成分,可选择与其他抗病毒药物、免疫调节剂、抗生素或疫苗组合使用。公开号:WO2018039157A1
文献信息
-
Asymmetric Methoxyselenenylations with Chiral Selenium Electrophiles作者:Liwei Zhao、Zhong Li、Thomas WirthDOI:10.1002/ejoc.201101373日期:2011.12Very simple, chiral, non-racemic diselenides were prepared and their corresponding selenium electrophiles were used for the stereoselective functionalization of alkenes. The influence of different alkenes on the outcome of the selenenylation reaction was investigated.
-
Method and compositions for identifying anti-HIV therapeutic compounds申请人:GILEAD SCIENCES, INC.公开号:US20040121316A1公开(公告)日:2004-06-24Methods are provided for identifying anti-HIV therapeutic compounds substituted with carboxyl ester or phosphonate ester groups. Libraries of such compounds are screened optionally using the novel enzyme GS-7340 Ester Hydrolase. Compositions and methods relating to GS-7340 Ester Hydrolase also are provided.
-
Synthetic, Structural, Mechanistic, and Computational Studies on Single-Site β-Diketiminate Tin(II) Initiators for the Polymerization of <i>r</i><i>ac</i>-Lactide作者:Andrew P. Dove、Vernon C. Gibson、Edward L. Marshall、Henry S. Rzepa、Andrew J. P. White、David J. WilliamsDOI:10.1021/ja061400a日期:2006.8.1showed that the lactate ligand forms a five-membered chelate ring with a weak donor bond from the carbonyl oxygen atom to the tin center. A B3LYP density functional computational study indicates that insertion of the first lactide monomer into the tin(II) alkoxide bond is facile, with the induction period arising from a slower insertion of the second (and possibly third and fourth) monomer units.由 β-二酮亚胺配体支持的锡 (II) 配合物家族已被研究作为 rac-丙交酯聚合的引发剂。动力学研究揭示了对 [丙交酯] 的一级依赖性,但具有显着的诱导期。M(n) 与转化率和 [M](o)/[I](o) 与转化率的线性图,以及窄的分子量分布(通常为 1.07-1.10)表明控制良好的“活性”聚合. 空间位阻较小的衍生物比其庞大的类似物促进更快的传播,与更容易接近的活性位点相一致。对于带有卤代 N-芳基取代基的配体,观察到聚合速率提高,这是 Sn(II) 中心更具路易斯酸性的结果。所有的引发剂都表现出对异同立构聚丙交酯的相似偏向,这归因于主要受 Sn 5s(2) 孤对电子的存在而不是 β-二酮亚胺配体的空间或电子特性影响的链端控制机制。锡 (II) 异丙基-(S)-乳酸络合物 ((Me)BDI(DIPP))SnOCH(Me)COO(i)Pr (14),已通过处理((Me)BDI(DIPP))Sn(NMe(2))
-
Organosoluble zirconium phosphonate nanocomposites and their supported chiral ruthenium catalysts: The first example of homogenization of inorganic-supported catalyst in asymmetric hydrogenation作者:Taotao Chen、Xuebing Ma、Xiaojia Wang、Qiang Wang、Jinqin Zhou、Qian TangDOI:10.1039/c0dt00786b日期:——In this article, we report the synthesis, structure, morphologies, and asymmetric catalytic properties of a series of novel organosoluble zirconium phosphonate nanocomposites and their supported chiral ruthenium catalysts, which have a good organosolubility (0.1–0.5 g mL−1) in various solvents and mesoporous, filiform, and layered structures. Due to the organosoluble properties in various organic solvents在本文中,我们报告了一系列新型有机可溶物的合成,结构,形态和不对称催化性能 磷酸锆纳米复合材料及其负载的手性钌催化剂,在各种溶剂和介孔,丝状和层状结构中均具有良好的有机溶解度(0.1–0.5 g mL -1)。由于在各种有机溶剂中的有机可溶特性,在催化领域实现了膦酸锆负载的催化剂的第一次均质化。在取代的α-酮酯的不对称氢化中,对映选择性(74.3–84.7%ee)和分离的产率(86.7–93.6%)高于相应的均相Ru(p -cymene)(S -BINAP)Cl 2由于膦酸锆骨架中剩余的中孔引起的限制作用。反应完成后,通过添加可轻松以定量收率回收负载型催化剂环己烷 离心后再重复使用五次,而催化活性没有明显损失。
-
Photochemical Synthesis of Prochiral Dialkyl 3,3-Dialkylcyclopropene-1,2-dicarboxylates with Facial Shielding Substituents and Related Substrates作者:A. Stephen K. Hashmi、Marc A. Grundl、Andreas Rivas Nass、Frank Naumann、Jan W. Bats、Michael BolteDOI:10.1002/1099-0690(200112)2001:24<4705::aid-ejoc4705>3.0.co;2-j日期:2001.12types of cyclopropene-1,2-dicarboxylates 1 have been obtained by photochemical methods from the corresponding pyrazoles 11, 12, or 13. These pyrazoles were synthesized by 1,3-dipolar cycloadditions of alkynes 8 or 9 with either preformed diazoalkanes or diazoalkanes generated photochemically in situ, by use of oxadiazolines as diazoalkane precursors. The numerous substrates have clearly established the
表征谱图
-
氢谱1HNMR
-
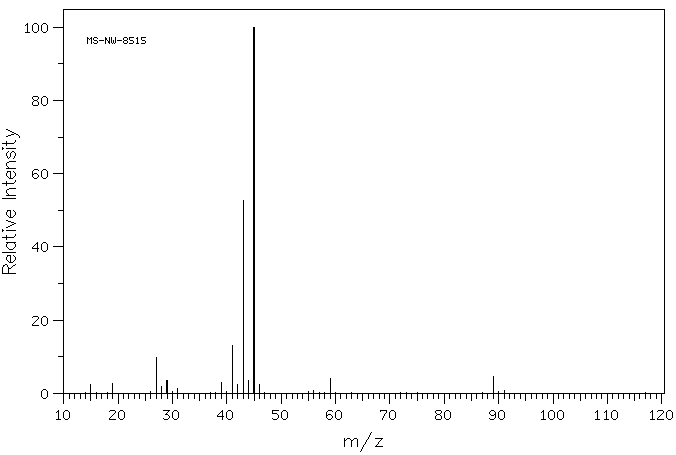
质谱MS
-
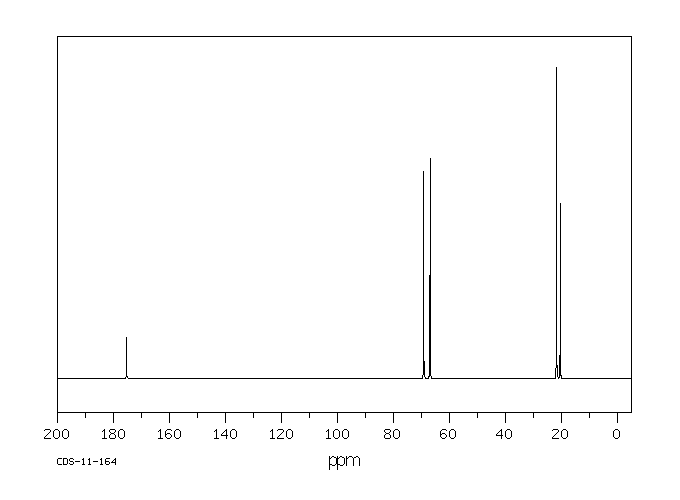
碳谱13CNMR
-
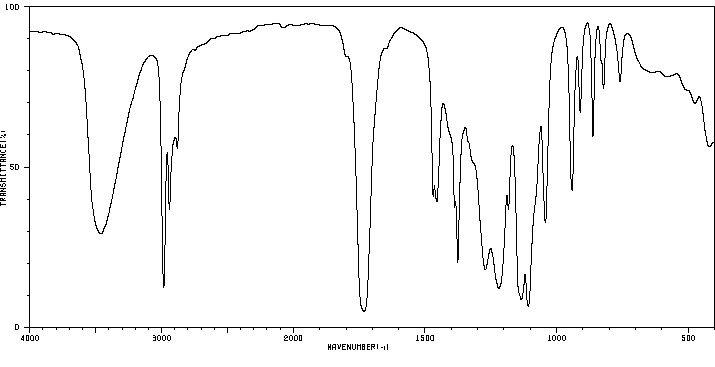
红外IR
-
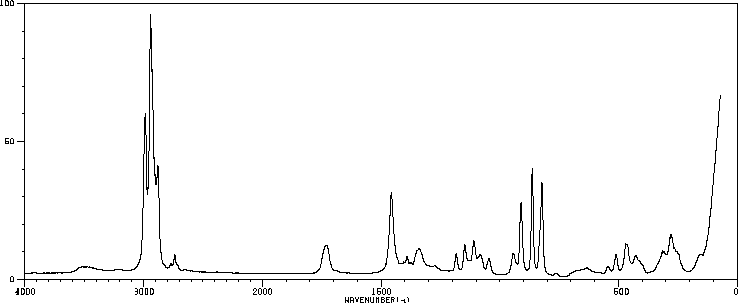
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸