1,2-联苯硫基乙烷 | 622-20-8
中文名称
1,2-联苯硫基乙烷
中文别名
1.2-二苯基硫代乙烷;1,2-双(苯硫基)乙烷
英文名称
1,2-bis(phenylthio)ethane
英文别名
2-phenylsulfanylethylsulfanylbenzene
CAS
622-20-8
化学式
C14H14S2
mdl
MFCD00014085
分子量
246.397
InChiKey
MHCVYAFXPIMYRD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:68 °C
-
沸点:65 °C / 3mmHg
-
密度:1.16±0.1 g/cm3(Predicted)
-
LogP:5.27 at 30℃
-
稳定性/保质期:
远离氧化物。
计算性质
-
辛醇/水分配系数(LogP):4.6
-
重原子数:16
-
可旋转键数:5
-
环数:2.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:50.6
-
氢给体数:0
-
氢受体数:2
安全信息
-
TSCA:Yes
-
危险等级:STENCH
-
安全说明:S22,S24/25
-
海关编码:2930909090
-
危险性防范说明:P264,P280,P302+P352+P332+P313+P362+P364,P305+P351+P338+P337+P313
-
危险性描述:H315,H319
-
储存条件:应将物品存放在密封容器中,并放置在阴凉、干燥的地方。请确保存储位置远离氧化剂。
SDS
1.1 产品标识符
: 1,1'-[Ethane-1,2-diylbis(thio)]bisbenzene
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
皮肤刺激 (类别2)
眼刺激 (类别2A)
慢性水生毒性 (类别4)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H413 可能对水生生物造成长期持续有害影响。
警告申明
预防
P264 操作后彻底清洁皮肤。
P273 避免释放到环境中。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
措施
P302 + P352 如与皮肤接触,用大量肥皂和水冲洗受感染部位.
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P321 具体治疗(见本标签上提供的急救指导)。
P332 + P313 如发生皮肤刺激:求医/ 就诊。
P337 + P313 如仍觉眼睛刺激:求医/就诊。 如仍觉眼睛刺激:求医/就诊.
P362 脱掉沾染的衣服,清洗后方可重新使用。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C14H14S2
分子式
: 246.39 g/mol
分子量
组分 浓度或浓度范围
1,1'-[Ethane-1,2-diylbis(thio)]bisbenzene
-
CAS 号 622-20-8
EC-编号 210-723-5
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 硫氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止粉尘的生成。 防止吸入蒸汽、气雾或气体。 保证充分的通风。 避免吸入粉尘。
6.2 环境保护措施
在确保安全的前提下,采取措施防止进一步的泄漏或溢出。 不要让产物进入下水道。
防止排放到周围环境中。
6.3 抑制和清除溢出物的方法和材料
收集、处理泄漏物,不要产生灰尘。 扫掉和铲掉。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止粉尘和气溶胶生成。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
按照良好工业和安全规范操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
辛醇--水的分配系数的对数值: 4.924
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
: 1,1'-[Ethane-1,2-diylbis(thio)]bisbenzene
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
皮肤刺激 (类别2)
眼刺激 (类别2A)
慢性水生毒性 (类别4)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H413 可能对水生生物造成长期持续有害影响。
警告申明
预防
P264 操作后彻底清洁皮肤。
P273 避免释放到环境中。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
措施
P302 + P352 如与皮肤接触,用大量肥皂和水冲洗受感染部位.
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P321 具体治疗(见本标签上提供的急救指导)。
P332 + P313 如发生皮肤刺激:求医/ 就诊。
P337 + P313 如仍觉眼睛刺激:求医/就诊。 如仍觉眼睛刺激:求医/就诊.
P362 脱掉沾染的衣服,清洗后方可重新使用。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C14H14S2
分子式
: 246.39 g/mol
分子量
组分 浓度或浓度范围
1,1'-[Ethane-1,2-diylbis(thio)]bisbenzene
-
CAS 号 622-20-8
EC-编号 210-723-5
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 硫氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止粉尘的生成。 防止吸入蒸汽、气雾或气体。 保证充分的通风。 避免吸入粉尘。
6.2 环境保护措施
在确保安全的前提下,采取措施防止进一步的泄漏或溢出。 不要让产物进入下水道。
防止排放到周围环境中。
6.3 抑制和清除溢出物的方法和材料
收集、处理泄漏物,不要产生灰尘。 扫掉和铲掉。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止粉尘和气溶胶生成。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
按照良好工业和安全规范操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
辛醇--水的分配系数的对数值: 4.924
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 苯基溴代乙基硫醚 1-Bromo-2-phenylthioethane 4837-01-8 C8H9BrS 217.129 2-苯硫基乙醇 2-(phenylthio)ethanol 699-12-7 C8H10OS 154.233 2-氯乙基苯基硫醚 2-Chloroethyl phenyl sulfide 5535-49-9 C8H9ClS 172.678 茴香硫醚 methyl-phenyl-thioether 100-68-5 C7H8S 124.207 1,2-二苯亚磺酰基乙烷 1,2-Bis(phenylsulfinyl)ethane 6099-21-4 C14H14O2S2 278.396 苯基乙烯基硫醚 phenylthioethylene 1822-73-7 C8H8S 136.218 苯基硫氰酸酯 phenyl thiocyanate 5285-87-0 C7H5NS 135.189 氯甲基苯硫醚 phenylthiomethyl chloride 7205-91-6 C7H7ClS 158.652 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— hexyl phenyl sulfide 943-78-2 C12H18S 194.341 1,2-二苯亚磺酰基乙烷 1,2-Bis(phenylsulfinyl)ethane 6099-21-4 C14H14O2S2 278.396 —— (meso)-1,2-bis(phenylsulfinyl)ethane 21461-90-5 C14H14O2S2 278.396 —— 1,2-(R,R)-bis-benzenesulfinyl-ethane 6099-21-4 C14H14O2S2 278.396 苯基乙烯基硫醚 phenylthioethylene 1822-73-7 C8H8S 136.218
反应信息
-
作为反应物:描述:参考文献:名称:通过烃氧化进行大环内酯化摘要:据报道,一种新型 Pd/亚砜催化的线性 ω- 烯酸的大环内酯化反应通过连续配体催化的烯丙基 CH 氧化进行。这种大内酯化的范围似乎非常广泛。芳基、烷基和 (Z)-α,β-不饱和酸都是该反应的亲核试剂,后者进行大环内酯化,没有烯烃异构化。观察到高官能团相容性,包括生物学和医学相关的功能,如邻位取代的水杨酸酯、双(吲哚)马来酰亚胺和肽。提供的证据支持这样的假设,即大环内酯化通过模板化的 pi-烯丙基钯羧酸中间体的内球官能化进行。DOI:10.1021/ja063096r
-
作为产物:描述:参考文献:名称:The Formation of α-Chloro Sulfides from Sulfides and from Sulfoxides摘要:DOI:10.1021/ja01608a016
-
作为试剂:描述:参考文献:名称:烯丙基C的硫化物配体研究。末端烯烃的H氧化摘要:完美的THT!筛选多样化的硫醚配体库导致发现四氢噻吩(THT)作为Pd催化的烯丙基CH氧化反应的高反应性和选择性配体(参见方案)。这种新颖的配体体系为通过烯丙基CH活化化学(BQ = 1,4-苯醌)形成(E)-线性烯丙基乙酸酯提供了某些报道的最高收率。DOI:10.1002/chem.201301787
文献信息
-
Catalytic Enantioselective Oxidation of Bulky Alkyl Aryl Thioethers with H2O2 over Titanium-Salan Catalysts作者:Konstantin P. Bryliakov、Evgenii P. TalsiDOI:10.1002/ejoc.201100557日期:2011.8procedure for the oxidation of bulky (preferably aryl benzyl substituted) thioethers with hydrogen peroxide in good to high yields and enantioselectivities (up to 98.5 % ee) is reported. The high optical yields are achieved in a tandem stereoconvergent enantioselective oxidation and kinetic resolution process. A reasonable balance between the sulfoxide yield and enantioselectivity could be found by varying
-
A highly efficient heterogeneous rhodium(I)-catalyzed C-S coupling reaction of thiols with polychloroalkanes or alkyl halides under mild conditions作者:Jianhui Xia、Ruiya Yao、Mingzhong CaiDOI:10.1002/aoc.3273日期:2015.4Heterogeneous C–S coupling reaction of thiols with polychloroalkanes or alkyl halides was achieved at 30 or 80 °C in the presence of 5 mol% of an MCM‐41‐immobilized bidentate phosphine rhodium complex (MCM‐41‐2P‐RhCl(PPh3)) and triethylamine, yielding a variety of formaldehyde dithioacetals, ethylenedithioethers and unsymmetric thioethers in good to excellent yields. This heterogeneous rhodium catalyst
-
A highly efficient carbon–sulfur bond formation reaction via microwave-assisted nucleophilic substitution of thiols to polychloroalkanes without a transition-metal catalyst作者:Yi-Ju Cao、Yuan-Yuan Lai、Hong Cao、Xiao-Ning Xing、Xiang Wang、Wen-Jing XiaoDOI:10.1139/v06-151日期:2006.11.1
An efficient carbon–sulfur bond formation reaction has been developed under microwave irradiation. This reaction affords a novel and rapid synthesis of thioacetals and sulfides under mild conditions. This method is particularly noteworthy given its experimental simplicity and high generality, and no transition-metal catalysts were needed under our conditions.Key words: microwave, sulfide, thiol, nucleophilic substitution.
-
Silica-promoted facile synthesis of thioesters and thioethers: a highly efficient, reusable and environmentally safe solid support作者:Basudeb Basu、Susmita Paul、Ashis K. NandaDOI:10.1039/b925620b日期:——An efficient, mild and rapid procedure for the acylation and alkylation of aromatic and aliphatic thiols mediated on a silica gel surface at room temperature is described. The protocol allows the protection of thiols under neutral heterogeneous conditions without requiring any bases or Lewis acids, and the silica gel used as the promoter can be recycled for several runs without any loss of activity.
-
Reactions of sulfinyl carbanions with organometallic compounds: Preparation of (η5-C5H5)2T6H4作者:C.R. LucasDOI:10.1016/s0022-328x(00)86902-8日期:1982.10have been observed to have often low reactivity towards neutral transition metal organometallic systems. When reactions do occur they usually involve salt eliminations and formation of complex mixtures containing inter alia organic sulfides, disulfides, freed ligands or their oxides and reduced forms of the organometallic reactants. From the sulfinyl ylide [PhS(O)CH2]Li, exo-[η5-C6H6CH2S(O)Ph]Mn(CO)3已观察到亚磺酰基碳负离子[RS(O)CHR'] - Li +(R = Ph,Me; R'= H,Ph)对中性过渡金属有机金属体系的反应活性通常较低。当确实发生反应时,它们通常涉及除盐和形成复杂混合物,所述混合物尤其包含有机硫化物,二硫化物,游离的配体或其氧化物以及还原形式的有机金属反应物。从亚磺酰基叶立德[PHS(O)CH 2 ]栗,外切- [η 5 -C 6 ħ 6 CH 2 S(O)PH]的Mn(CO)3和新metallocyclic化合物(η 5 -C 5 H ^ 5)2TICH 2 SC 6 H ^ 4已经制备,检查后者的一些性质和它的锍衍生物[(η 5 -C 5 H ^ 5)2 TiCH2S(ME)C6H 4 ] BF 4隔离。
表征谱图
-
氢谱1HNMR
-
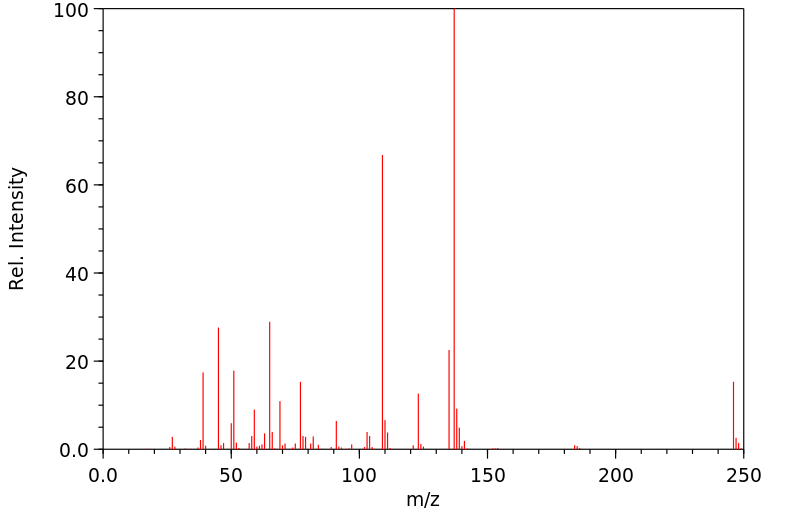
质谱MS
-
碳谱13CNMR
-
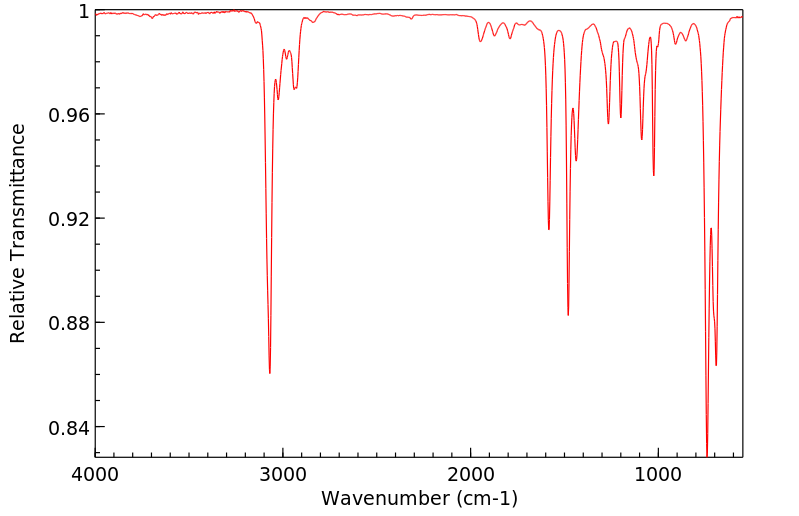
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Rp)-2-(叔丁硫基)-1-(二苯基膦基)二茂铁
(1E)-1-{4-[(4-氨基苯基)硫烷基]苯基}乙酮肟
颜料红88
颜料紫36
顺式-1,2-二(乙硫基)-1-丙烯
非班太尔-D6
雷西那得中间体
阿西替尼杂质J
阿西替尼杂质C
阿西替尼杂质4
阿西替尼杂质
阿西替尼
阿拉氟韦
阿扎毒素
阿嗪米特
阔草特
银(I)(6-氨基-2-(甲硫基)-5-亚硝基嘧啶-4-基)酰胺水合物
钾三氟[3-(苯基硫基)丙基]硼酸酯(1-)
邻甲苯基(对甲苯基)硫化物
避虫醇
连翘脂苷B
还原红 41
还原紫3
还原桃红R
达索尼兴
辛硫醚
辛-1,7-二炔-1-基(苯基)硫烷
西嗪草酮
萘,2-[(2,3-二甲基苯基)硫代]-
莫他哌那非
茴香硫醚
苯醌B
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-甲基苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2,6-二氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,2-[(2-硝基苯基)硫代]-
苯酚,3-氯-4-[(4-硝基苯基)硫代]-
苯酚,3-(乙硫基)-
苯酚,3,5-二[(苯基硫代)甲基]-
苯胺,4-[5-溴-3-[4-(甲硫基)苯基]-2-噻嗯基]-
苯胺,3-氯-4-[(1-甲基-1H-咪唑-2-基)硫代]-
苯胺,2-[(2-吡啶基甲基)硫代]-
苯硫醚-D10
苯硫胍
苯硫基乙酸
苯硫代磺酸S-(三氯乙烯基)酯
苯甲醇,2,3,4,5,6-五氟-a-[(苯基硫代)甲基]-,(R)-
苯甲酸,3-[[2-[(二甲氨基)甲基]苯基]硫代]-,盐酸
苯甲胺,5-氟-2-((3-甲氧苯基)硫代)-N,N-二甲基-,盐酸
苯甲二硫酸,4-溴苯基酯