苯基溴代乙基硫醚 | 4837-01-8
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:140 °C
-
密度:1,44 g/cm3
-
闪点:140-141°C/10mm
-
稳定性/保质期:
在常温常压下,该物质是稳定的。
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:10
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:25.3
-
氢给体数:0
-
氢受体数:1
安全信息
-
安全说明:S26,S36
-
危险类别码:R36/38
-
海关编码:2930909090
-
危险性防范说明:P233,P260,P261,P264,P271,P280,P302+P352,P304,P304+P340,P305+P351+P338,P312,P321,P332+P313,P337+P313,P340,P362,P403,P403+P233,P405,P501
-
危险性描述:H315,H319,H335
-
储存条件:常温下应存放在避光、阴凉干燥处,并密封保存。
SDS
Section I.Chemical Product and Company Identification
Chemical Name 2-Bromoethyl Phenyl Sulfide
Portland OR
Synonym [(2-Bromoethyl)thio]-benzene
Chemical Formula C6H5SCH2Br
CAS Number 4837-01-8
Section II. Composition and Information on Ingredients
Chemical Name CAS Number Percent (%) TLV/PEL Toxicology Data
2-Bromoethyl Phenyl Sulfide 4837-01-8 ---------- Not available. Not available.
Section III. Hazards Identification
Acute Health Effects No specific information is available in our data base regarding the toxic effects of this material for humans. However,
exposure to any chemical should be kept to a minimum. Skin and eye contact may result in irritation. May be harmful if
inhaled or ingested. Always follow safe industrial hygiene practices and wear proper protective equipment when handling
this compound. Follow safe industrial hygiene practices and always wear proper protective equipment when handling this
compound.
Chronic Health Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions.
Section IV. First Aid Measures
Eye Contact Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15
minutes. Get medical attention.
Skin Contact In case of contact, immediately flush skin with plenty of water. Remove contaminated clothing and shoes. Wash clothing
before reuse. Thoroughly clean shoes before reuse. Get medical attention.
Inhalation If the victim is not breathing, perform mouth-to-mouth resuscitation. Loosen tight clothing such as a collar, tie, belt or
waistband. If breathing is difficult, oxygen can be administered. Seek medical attention if respiration problems do not
improve.
Ingestion INDUCE VOMITING by sticking finger in throat. Lower the head so that the vomit will not reenter the mouth and throat.
Loosen tight clothing such as a collar, tie, belt or waistband. If the victim is not breathing, perform mouth-to-mouth
resuscitation. Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the
toxic material was ingested; the absence of such signs, however, is not conclusive. SEEK IMMEDIATE MEDICAL
ATTENTION in case of ingestion of a radioactive material.
Section V. Fire and Explosion Data
Not available.
Flammability May be combustible at high temperature. Auto-Ignition
Flammable Limits
Flash Points Not available.
Not available.
Combustion Products These products are toxic carbon oxides (CO, CO 2), sulfur oxides (SO2, SO3...), halogenated compounds.
Fire Hazards
Not available.
Explosion Hazards Risks of explosion of the product in presence of mechanical impact: Not available.
Risks of explosion of the product in presence of static discharge: Not available.
Fire Fighting Media
SMALL FIRE: Use DRY chemical powder.
and Instructions LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet.
Consult with local fire authorities before attempting large scale fire-fighting operations.
Continued on Next Page
2-Bromoethyl Phenyl Sulfide
Section VI. Accidental Release Measures
Spill Cleanup Absorb with an inert material and put the spilled material in an appropriate waste disposal. Finish cleaning the spill by
Instructions rinsing any contaminated surfaces with copious amounts of water. Consult federal, state, and/or local authorities for
assistance on disposal.
Section VII. Handling and Storage
Handling and Storage Keep away from heat. Mechanical exhaust required. When not in use, tightly seal the container and store in a dry, cool
place. Avoid excessive heat and light. Do not breathe gas/fumes/ vapor/spray.
Information
Always store away from incompatible compounds such as oxidizing agents.
Section VIII. Exposure Controls/Personal Protection
Engineering Controls Provide exhaust ventilation or other engineering controls to keep the airborne concentrations of vapors below their
respective threshold limit value. Ensure that eyewash station and safety shower is proximal to the work-station location.
Personal Protection Splash goggles. Lab coat. Vapor respirator. Boots. Gloves. Suggested protective clothing might not be sufficient;
consult a specialist BEFORE handling this product. Be sure to use a MSHA/NIOSH approved respirator or equivalent.
Exposure Limits
Not available.
Section IX. Physical and Chemical Properties
Physical state @ 20°C Liquid. Solubility
Not available.
Not available.
Specific Gravity
Molecular Weight Partition Coefficient
217.13 Not available.
Boiling Point Vapor Pressure
Not available. Not available.
Melting Point Not available. Vapor Density Not available.
Refractive Index Not available. Volatility Not available.
Critical Temperature Not available. Odor Not available.
Viscosity Not available. Taste Not available.
Section X. Stability and Reactivity Data
Stability
This material is stable if stored under proper conditions. (See Section VII for instructions)
Conditions of Instability Avoid excessive heat and light.
Incompatibilities
Reactive with oxidizing agents.
Section XI. Toxicological Information
RTECS Number Not available.
Routes of Exposure Eye Contact. Ingestion. inhalation.
Toxicity Data Not available.
CARCINOGENIC EFFECTS : Not available.
Chronic Toxic Effects
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions.
Acute Toxic Effects No specific information is available in our data base regarding the toxic effects of this material for humans. However,
exposure to any chemical should be kept to a minimum. Skin and eye contact may result in irritation. May be harmful if
inhaled or ingested. Always follow safe industrial hygiene practices and wear proper protective equipment when handling
this compound. Follow safe industrial hygiene practices and always wear proper protective equipment when handling this
compound.
Continued on Next Page
2-Bromoethyl Phenyl Sulfide
Section XII. Ecological Information
Ecotoxicity Not available.
Environmental Fate Not available.
Section XIII. Disposal Considerations
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissove or mix material with a
Waste Disposal
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all
federal, state and locl regulations when disposing of the substance.
Section XIV. Transport Information
DOT Classification Not a DOT controlled material (United States).
PIN Number Not applicable.
Proper Shipping Name
Not applicable.
Packing Group (PG) Not applicable.
DOT Pictograms
Section XV. Other Regulatory Information and Pictograms
TSCA Chemical Inventory This product is NOT on the EPA Toxic Substances Control Act (TSCA) inventory. The following notices are required by 40
CFR 720.36 (C) for those products not on the inventory list:
(EPA)
(i) These products are supplied solely for use in research and development by or under the supervision of a technically
qualified individual as defined in 40 CFR 720.0 et sec.
(ii) The health risks of these products have not been fully determined. Any information that is or becomes available will be
supplied on an MSDS sheet.
WHMIS Classification Not available.
(Canada)
EINECS Number (EEC) 225-422-4
EEC Risk Statements Not available.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
聚合物和大分子半导体砌块
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-苯硫基乙醇 2-(phenylthio)ethanol 699-12-7 C8H10OS 154.233 2-氯乙基苯基硫醚 2-Chloroethyl phenyl sulfide 5535-49-9 C8H9ClS 172.678 苯硫基乙酸 (phenylthio)acetic acid 103-04-8 C8H8O2S 168.216 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-(苯基硫代)乙胺盐酸盐 2-(phenylthio)ethanamine 2014-75-7 C8H11NS 153.248 2-苯硫基乙醇 2-(phenylthio)ethanol 699-12-7 C8H10OS 154.233 1,2-联苯硫基乙烷 1,2-bis(phenylthio)ethane 622-20-8 C14H14S2 246.397 N-甲基-2-(苯基硫基)乙胺 N-methyl-2-(phenylthio)ethylamine 2014-78-0 C9H13NS 167.275 —— (2-Phenylthio-aethyl)-hydrazin 55459-98-8 C8H12N2S 168.263 —— isopropenyl phenyl sulfide 7594-43-6 C9H10S 150.244 —— (2-azidoethyl)(phenyl)sulfane 106118-84-7 C8H9N3S 179.246 —— 2-bromoethyl phenyl sulfoxide 193097-47-1 C8H9BrOS 233.129 —— 2-(Phenylsulfanyl)ethyl nitrite 112752-00-8 C8H9NO2S 183.231 —— (2-Phenylsulfanyl-ethyl)-propyl-amine 7319-97-3 C11H17NS 195.329 —— 2-Phenylthio-1-(2-hydroxyethylamino)-ethan 59264-36-7 C10H15NOS 197.301 苯基乙烯基硫醚 phenylthioethylene 1822-73-7 C8H8S 136.218 —— 2-phenylthio-1-nitroethane 52809-70-8 C8H9NO2S 183.231 —— N,N-Diethyl-2-phenylthioethylamine 6006-18-4 C12H19NS 209.356 —— N1,N1-dimethyl-N2-(2-(phenylthio)ethyl)ethane-1,2-diamine —— C12H20N2S 224.37 —— 4-phenylthio-2-methoxycarbonyl-butanoate 128561-98-8 C11H16O2S 212.313 - 1
- 2
反应信息
-
作为反应物:描述:苯基溴代乙基硫醚 在 sodium hydride 、 三乙胺 作用下, 以 四氢呋喃 为溶剂, 生成 S-(2-phenothiazin-10-yl-ethyl)-S-phenyl-N-(toluene-4-sulfonyl)-sulfimide参考文献:名称:乙烯基磺胺的迈克尔型反应的研究摘要:合成了 S-苯基-S-乙烯基-N-甲苯磺酰基-硫亚胺 (3) 并研究了 (3) 与各种亲核试剂如硫醇、二硫代氨基甲酸酯、醇、胺和丙二腈的 Michael 型反应。得到的加合物(4)热解得到乙烯基化合物(7)和次磺酰胺(8),收率良好。DOI:10.1246/cl.1975.581
-
作为产物:描述:参考文献:名称:铟(III)催化一价羧酸从羧酸中合成烷基氰化物摘要:摘要 描述了通过烷基碘化物或烷基溴从羧酸一锅制备烷基氰化物,其通过铟(III)催化的羧酸的还原碘化或溴化反应原位生成。 描述了通过烷基碘化物或烷基溴从羧酸一锅制备烷基氰化物,其通过铟(III)催化的羧酸的还原碘化或溴化反应原位生成。DOI:10.1055/s-0033-1339903
文献信息
-
Potential hypnotics and anxiolytics in the 4H-s-triazolo[4,3-a]-1,4-benzodiazepine series: 8-Chloro-6-(2-chlorophenyl)-1-[4-(2-methoxyethyl)piperazino]-4H-s-triazolo[4,3-a]-1,4-benzodiazepine and some related compounds作者:Zdeněk Polívka、Miroslav Ryska、Jiří Holubek、Emil Svátek、Jan Metyš、Miroslav ProtivaDOI:10.1135/cccc19832395日期:——
1-Bromo derivatives
XIIa andXIIb were prepared by bromination of 8-chloro-6-phenyl-4H -s -triazolo[4,3-a ]-1,4-benzodiazepine (XIa ) and its 6-(2-chlorophenyl) analogueXIb with bromine in chloroform in the presence of pyridine. Substitution reactions with 1-(2-methoxyethyl)piperazine (XIVb ), 1-(3-methoxypropyl)piperazine (XVb ), 1-(2-ethoxyethyl)piperazine (XVIb ) and 1-(2-methylthioethyl)piperazine (XVIIb ) afforded the title compoundIIb and analoguesIIa andIIIb-Vb . A substitution reaction of the bromo derivativeXIIb with piperazine gave the 1-piperazino derivativeVIIIb which was alkylated with 2-phenoxyethyl bromide and 2-phenylthioethyl bromide to give compoundsVIb andVIIb . The title compoundIIb has very high anticonvulsant and discoordinating activities in mice. The enlargement of the substituent R1 (compoundsIIIb-VIIb ) results in a gradual decrease of the effects mentioned.1-溴衍生物XIIa和XIIb是通过在氯仿中存在吡啶的情况下,用溴对8-氯-6-苯基-4H-s-三唑并[4,3-a]-1,4-苯二氮杂环己烷(XIa)及其6-(2-氯苯基)类似物XIb进行溴化制备的。用1-(2-甲氧基乙基)哌嗪(XIVb)、1-(3-甲氧基丙基)哌嗪(XVb)、1-(2-乙氧基乙基)哌嗪(XVIb)和1-(2-甲硫基乙基)哌嗪(XVIIb)进行取代反应,得到了标题化合物IIb及其类似物IIa和IIIb-Vb。溴衍生物XIIb与哌嗪的取代反应得到了1-哌嗪基衍生物VIIIb,它与2-苯氧基乙溴和2-苯硫基乙溴烷基化,得到化合物VIb和VIIb。标题化合物IIb在小鼠中具有非常高的抗惊厥和失调活性。取代基R1的扩大(化合物IIIb-VIIb)导致上述效应逐渐减弱。 -
Design, synthesis, and activity evaluation of selective inhibitors of anti-apoptotic Bcl-2 proteins: The effects on the selectivity of the P1 pockets in the active sites作者:Mingping Wang、Wei Tian、Chongqing Wang、Shihai Lu、Chao Yang、Juan Wang、Yunlong Song、Youjun Zhou、Ju Zhu、Zhiyu Li、Canhui ZhengDOI:10.1016/j.bmcl.2016.09.061日期:2016.11The anti-apoptotic Bcl-2 proteins are attractive targets for anti-cancer drug development, and the discovery of their selective inhibitors has become a research focus. In this Letter, obvious differences in the P1 pocket of the active site between Bcl-2, Bcl-xL, and Mcl-1 proteins were proposed by the structural comparison of these proteins. As a result, the groups in their inhibitors binding to the P1
-
Preparation of Tetraalkylammonium<i>N</i>-Chloro-<i>p</i>-toluenesulfonamides and Their Application to Imination of Phosphorus Compounds and Sulfides作者:Tamotsu Yamamoto、Daisaburo Yoshida、Jun Hojyo、Hiranari TerauchiDOI:10.1246/bcsj.57.3341日期:1984.11Tetraalkylammonium N-chloro-p-toluenesulfonamides were prepared from chloramine T and tetraalkylammonium chlorides as a substance corresponding to anhydrous chloramine T, and found to give rise to the effective tosylimination of phosphorus compounds and diaryl sulfides.
-
Preparation of<i>S</i>-Vinylsulfilimines作者:Tamotsu Yamamoto、Masa-aki Kakimoto、Makoto OkawaraDOI:10.1246/bcsj.52.841日期:1979.3For the preparation of S-vinylsulfilimines (1), mainly S-phenyl-N-tosyl-S-vinylsulfilimine, three routes were examined, viz., (A) the reaction of vinyl sulfide with chloramine T, (B) that of 2-haloethyl phenyl sulfide with chloramine T followed by dehydrohalogenation, and (C) that of 2-hydroxyethyl sulfide with chloramine T followed by acetylation of the hydroxyl group and deacetoxylation. Route A has disadvantages of troublesome preparation of vinyl sulfides and low yield. Route B gives the precursor sulfilimine to 1 in good yield only by use of anhydrous chloramine T. Route C gives the best results in view of starting material and yield.
-
Highly atom-economic, catalyst- and solvent-free oxidation of sulfides into sulfones using 30% aqueous H2O2作者:Marjan JerebDOI:10.1039/c2gc36073j日期:——Highly atom-efficient oxidation of sulfides into sulfones under solvent- and catalyst-free reaction conditions using a 30% aqueous solution of H2O2 at 75 °C is reported. A structurally diverse set of phenyl alkyl-, phenyl benzyl-, benzyl alkyl-, dialkyl-, heteroaryl alkyl- and cyclic sulfides were transformed into sulfones regardless of the aggregate state and electronic nature of the substituents原子效率高 氧化作用 的 硫化物 进入 砜类 在下面 溶剂- 和 催化剂报道了在75℃下使用30%的H 2 O 2水溶液的无反应条件。结构上多样化的一组苯基 烷基-, 苯基 苄基-, 苄基 烷基-,二烷基-, 杂芳基 烷基-和循环 硫化物 被转化为 砜类与取代基的聚集状态和电子性质无关。尽管整个工作过程中反应混合物均不均匀,但没有发现搅拌困难和反应进展的问题。在许多情况下,仅使用过量10 mol%的H 2 O 2,因此大大提高了该方法的高原子经济性。一些固体基材需要可变过量的过氧化氢; 但是,反应是严格进行的,没有有机物溶剂。事实证明,这种转变适合液体和固体放大硫化物。此外,隔离和纯化 的原油产品可以仅用 过滤 和 结晶。
表征谱图
-
氢谱1HNMR
-
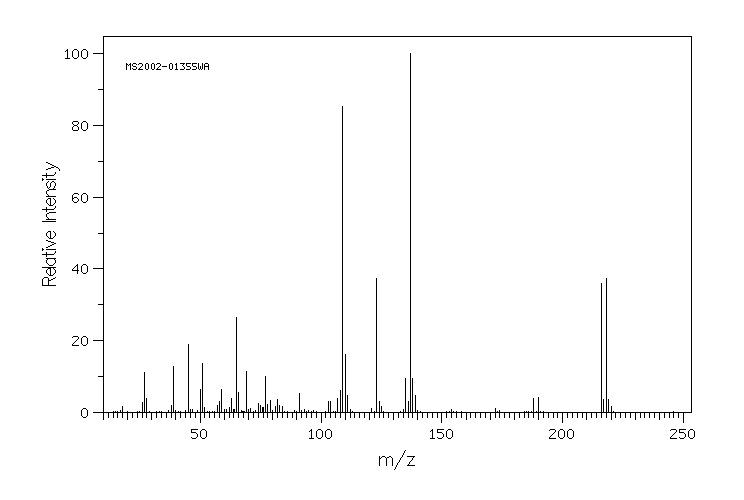
质谱MS
-
碳谱13CNMR
-
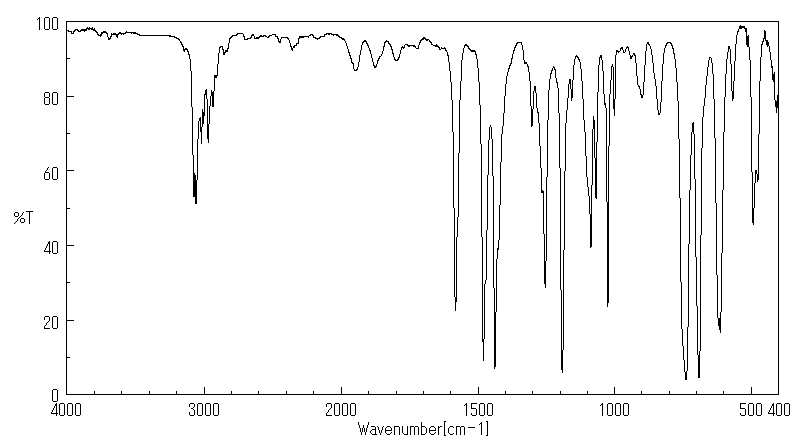
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息