3,6-二苯基-1,2,4,5-四嗪 | 6830-78-0
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:198 °C (dec.) (lit.)
-
沸点:446.0±28.0 °C(Predicted)
-
密度:1.214±0.06 g/cm3(Predicted)
-
溶解度:在氯仿的溶解度为25mg/mL,透明,深红色
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:18
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:51.6
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
海关编码:2933990090
-
储存条件:室温
SDS
模块 1. 化学品
1.1 产品标识符
: 3,6-二苯基-1,2,4,5-四嗪
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
皮肤刺激 (类别 2)
眼睛刺激 (类别 2A)
特异性靶器官系统毒性(一次接触) (类别 3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
警告申明
预防措施
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P264 操作后彻底清洁皮肤。
P271 只能在室外或通风良好之处使用。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
事故响应
P302 + P352 如果皮肤接触:用大量肥皂和水清洗。
P304 + P340 如吸入: 将患者移到新鲜空气处休息,并保持呼吸舒畅的姿势。
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P312 如感觉不适,呼救中毒控制中心或医生.
P321 具体处置(见本标签上提供的急救指导)。
P332 + P313 如觉皮肤刺激:求医/就诊。
P337 + P313 如仍觉眼睛刺激:求医/就诊。
P362 脱掉沾污的衣服,清洗后方可再用。
安全储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P405 存放处须加锁。
废弃处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C14H10N4
分子式
: 234.26 g/mol
分子量
组分 浓度或浓度范围
3,6-Diphenyl-1,2,4,5-tetrazine
<=100%
化学文摘登记号(CAS 6830-78-0
No.)
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
使用个人防护用品。 避免粉尘生成。 避免吸入蒸气、烟雾或气体。 保证充分的通风。
人员疏散到安全区域。 避免吸入粉尘。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
收集和处置时不要产生粉尘。 扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免形成粉尘和气溶胶。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
完全接触
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
飞溅保护
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
, 测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不同于EN
374规定的条件下应用,请与EC批准的手套的供应商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 粉末
颜色: 红色, 紫色的
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 198 °C - 分解
f) 沸点、初沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
吸入 - 可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: XF6863300
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 3-(methylthio)-6-phenyl-1,2,4,5-tetrazine 58106-82-4 C9H8N4S 204.255 5-苯基四氮唑 5-Phenyl-1H-tetrazole 18039-42-4 C7H6N4 146.151 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3,6-bis(2-fluorophenyl)-1,2,4,5-tetrazine 58884-40-5 C14H8F2N4 270.241 四螨嗪 bisclofentezin 74115-24-5 C14H8Cl2N4 303.15 —— 3-(2,6-dichlorophenyl)-6-(2-chlorophenyl)-1,2,4,5-tetrazine 162320-81-2 C14H7Cl3N4 337.595 3,5-二(苯基)-1H-1,2,4-三唑 3,5-diphenyl-1,2,4-triazole 2039-06-7 C14H11N3 221.261
反应信息
-
作为反应物:描述:参考文献:名称:Reactions of Tetrazines with Unsaturated Compounds. A New Synthesis of Pyridazines摘要:DOI:10.1021/ja01525a060
-
作为产物:描述:参考文献:名称:将异腈-四嗪 (4+1) 环加成反应和 Ugi 四组分反应合并为单一多组分工艺摘要:开发了结合异腈-四嗪(4+1)环加成和Ugi四组分反应特点的单一多组分工艺。多组分反应利用了第一步的点击反应性,并提供了迄今为止无法进入的吡唑酰胺衍生物的化学空间。DOI:10.1002/anie.202311186
-
作为试剂:描述:9,10-dihydro-10-methylacridine 在 3,6-二苯基-1,2,4,5-四嗪 、 水 、 scandium tris(trifluoromethanesulfonate) 作用下, 以 乙腈 为溶剂, 生成 N-甲基吖啶参考文献:名称:钪离子促进杂环神经网络双键的还原。氢化物转移与电子转移摘要:氢化物从 10-甲基-9,10-二氢吖啶 (AcrH(2)) 转移到 3,6-二苯基-1,2,4,5-四嗪 (Ph(2)Tz),其中包含一个 N=N 双键, 在 Sc(OTf)(3) (OTf = OSO(2)CF(3)) 存在于 298 K 的脱气乙腈 (MeCN) 中时有效发生,而在没有 Sc(3+) 的情况下不发生反应。观察到的二阶速率常数 (k(obs)) 随 Sc(3+) 浓度的增加而增加,以接近有限的值。当 AcrH(2) 被双氘化化合物 (AcrD(2)) 取代时,Sc(3+) 促进的氢化物转移的速率表现出相同的主要动力学同位素效应 (k(H)/k(D) = 5.2+ /-0.2),与 Sc(3+) 浓度无关。钪离子还促进了从 CoTPP(TPP(2)(-) = 四苯基卟啉二价阴离子)和 10,10'-二甲基-9,9'-联吖啶 [(AcrH)(2)] 到 Ph(2)Tz 的电子转移,而在没有DOI:10.1021/ja026592y
文献信息
-
TETRAZINE-BASED BIO-ORTHOGONAL COUPLING REAGENTS AND METHODS申请人:Fox Joseph Michael公开号:US20090023916A1公开(公告)日:2009-01-22Coupling reactions, suitable for use in organic or aqueous media, are performed by contacting a 1,2,4,5-tetrazine with a dienophile. The dienophile may be covalently bonded to a protein, and the coupling reaction may be performed in biological media such as those containing cells or cell lysates. The reactions may be performed in the presence of primary amines, thiols, acetylenes, azides, phosphines, and products of Staudinger and/or Sharpless-Huisgen reactions Novel 3-substituted cyclopropene compounds and trans-cyclooctenes are exemplary dienophiles for these reactions.
-
1,2-Diazacyclopentane-3,5-diyl Diradicals: Electronic Structure and Reactivity作者:Shohei Yoshidomi、Manabu AbeDOI:10.1021/jacs.8b12254日期:2019.3.6new series of long-lived singlet diradicals, viz., 1,2-diazacyclopentane-3,5-diyl, were identified, and their electronic structures and novel reactivities were thoroughly studied using laser-flash photolysis (LFP), product analysis, and computational studies. A direct observation of the thermal equilibration (fast process) between the singlet diradicals and the corresponding ring-closing compounds was局部单线态双自由基是键均裂的关键中间体。需要对活性物质进行彻底的研究,以阐明均裂键断裂和形成过程的机制。一般来说,单线态双自由基的寿命很短,因为自由基-自由基偶联反应很快。短暂的特性阻碍了对键均裂的深入研究。在这项研究中,鉴定了一系列新的长寿命单线态双自由基,即 1,2-二氮杂环戊烷-3,5-二基,并使用激光闪光光解 (LFP) 彻底研究了它们的电子结构和新型反应性、产品分析和计算研究。在亚微秒时间尺度上对单线态双自由基和相应的闭环化合物之间的热平衡(快速过程)进行了直接观察。溶剂和取代基对闭环反应和开环反应的平衡常数和速率常数的影响阐明了新型氮原子对局部单线态 1,3-二基双自由基的影响。两种类型的烷氧基迁移化合物 9 和 10 作为最终产物以高产率分离。用于形成烷氧基迁移产物(即 9 对 10)的交叉、自旋捕获和 LFP 实验揭示了独特的温度对两种烷氧基迁移产物的产物比率的影响。对温度
-
Oxidation von Methylpiperidin-Derivaten unter Berücksichtigung der Chiralität / Oxidation of Methylpiperidine Derivatives with Regard to Chirality作者:Hans Möhrle、Thomas BerkenkemperDOI:10.1515/znb-2007-1208日期:2007.12.1
When 2-(2-methyl-1-piperidinyl)ethanol derivatives 3a and 3b were dehydrogenated with Hg(II)- EDTA, an iminium function involving the tertiary α-carbon atom of the piperidine ring is formed regioselectively. Cyclization of these intermediates yielded diastereomeric mixtures of oxazolidines 7a and 7b, in solutions of which hydroxy-enamine species 8a/9a and 8b/9b, respectively, could be detected by NMR spectroscopy. A hydroxy-enamine derived from 7a could be trapped by cycloaddition to tetrazine 10. Protonation of the oxazolidines generated the iminium salts 6a/6b・X with loss of a chirality center. For prevention of different directions of ring dehydrogenation in the 2-(3-methyl- 1-piperidinyl)ethanol compounds, the 6-position was blocked with two methyl groups. With amino alcohol 17, the isolation of one of the racemates in pure form was achieved, which by dehydrogenation produced a diastereoisomeric lactam mixture 18, as shown by NMR spectroscopy. Reaction of 2-(4-methyl-1-piperidinyl)ethanol 19 with Hg(II)-EDTA gave rise to a diastereomeric lactam mixture 21 in the ratio 60 : 40. From enantiomerically pure phenyloxiranes, the amino alcohols R(-)-19 and S(+)-19 became available. Their dehydrogenation under standardized conditions always showed a spreading range of isomeric lactams, which could not be separated.
当2-(2-甲基-1-哌啶基)乙醇衍生物3a和3b与Hg(II)-EDTA脱氢时,哌啶环的叔α-碳原子形成区域选择性的亚胺功能。这些中间体的环化产生了对映异构体混合物7a和7b的恶唑啉,通过NMR光谱学可以检测到溶液中的羟基烯胺物种8a/9a和8b/9b。来自7a的羟基烯胺可以通过与四嗪10的环加成反应捕获。恶唑啉的质子化生成了亚胺盐6a/6b・X,并失去了一个手性中心。为了防止2-(3-甲基-1-哌啶基)乙醇化合物中环的脱氢反应沿不同方向进行,第6位被两个甲基基团阻断。使用氨基醇17,实现了纯形式的一个对映异构体的分离,其脱氢反应生成了由NMR光谱学显示的立体异构体混合物18。2-(4-甲基-1-哌啶基)乙醇19与Hg(II)-EDTA反应产生对映异构体混合物21的比例为60:40。从对映体纯的苯基环氧烷获得了氨基醇R(-)-19和S(+)-19。在标准化条件下它们的脱氢反应总是显示出不能分离的异构内酰胺的广泛范围。 -
BIO-ORTHOGONAL DRUG ACTIVATION申请人:KONINKLIJKE PHILIPS N.V.公开号:US20160106859A1公开(公告)日:2016-04-21The invention relates to a Prodrug activation method, for therapeutics, wherein use is made of abiotic reactive chemical groups that exhibit bio-orthogonal reactivity towards each other. The invention also relates to a Prodrug kit comprising at least one Prodrug and at least one Activator, wherein the Prodrug comprises a Drug and a first Bio-orthogonal Reactive Group (the Trigger), and wherein the Activator comprises a second Bio-orthogonal Reactive Group. The invention also relates to targeted therapeutics used in the above-mentioned method and kit. The invention particularly pertains to antibody-drug conjugates and to bi- and trispecific antibody derivatives.
-
Reactions of relevance to the chemistry of aminoglycoside antibiotics. Part 13. A novel synthesis of benzyl ethers作者:Anthony G. M. Barrett、Roger W. Read、Derek H. R. BartonDOI:10.1039/p19800002184日期:——Benzyl ethers were prepared from alcohols by reaction with chloro(phenylmethylene)dimethylammonium chloride and sodium hydrogen telluride in sequence. The salt (1)[Me2NC(R1)OR2Cl–; R1= H, R2= cholest-5-en-3β-yl] and sodium borohydride gave the borane complex of 3β-dimethylaminomethoxycholest-5-ene. Salt (1; R1= Ph, R2= cholest-5-en-3β-yl or 5α-cholestan-3β-yl) and ammonia or hydrazine gave the steroidal
表征谱图
-
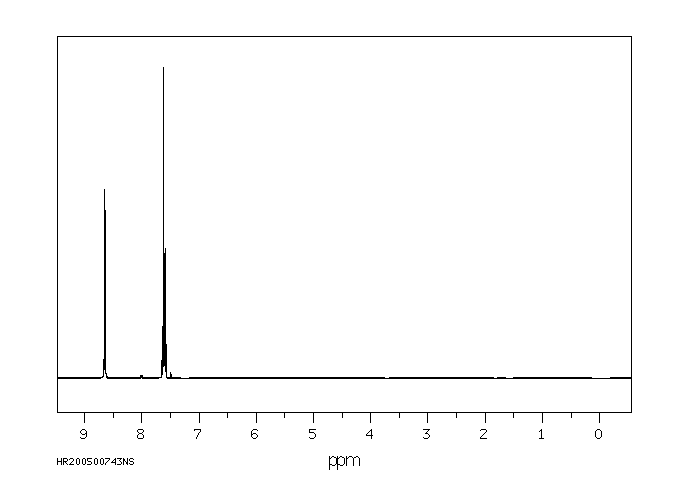
氢谱1HNMR
-
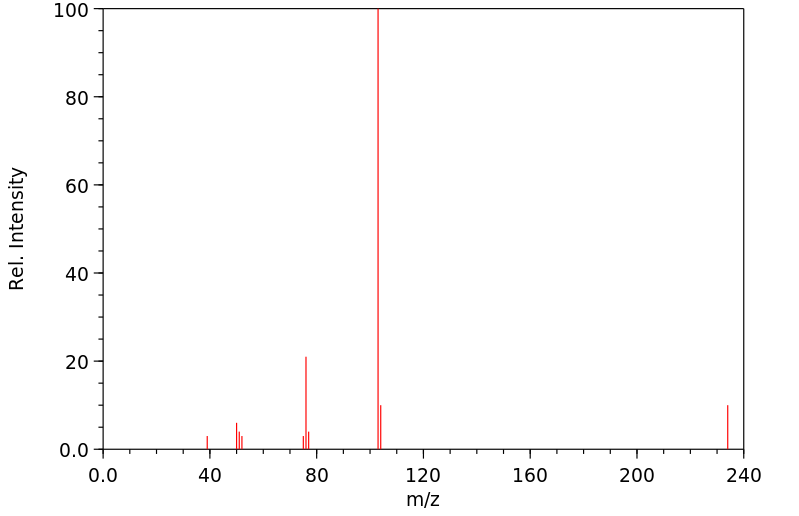
质谱MS
-
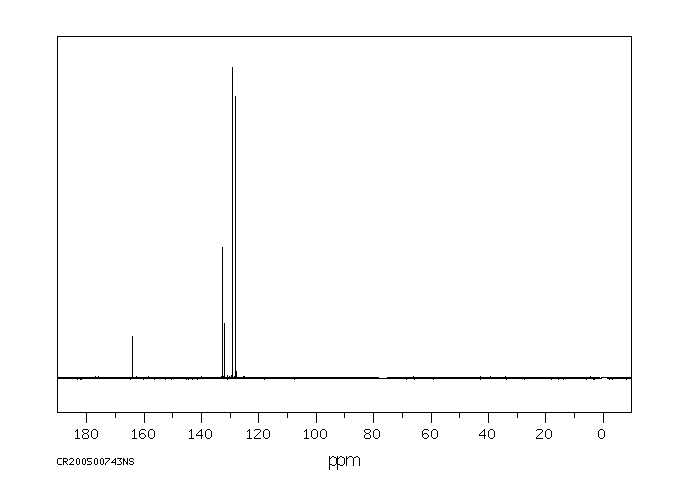
碳谱13CNMR
-
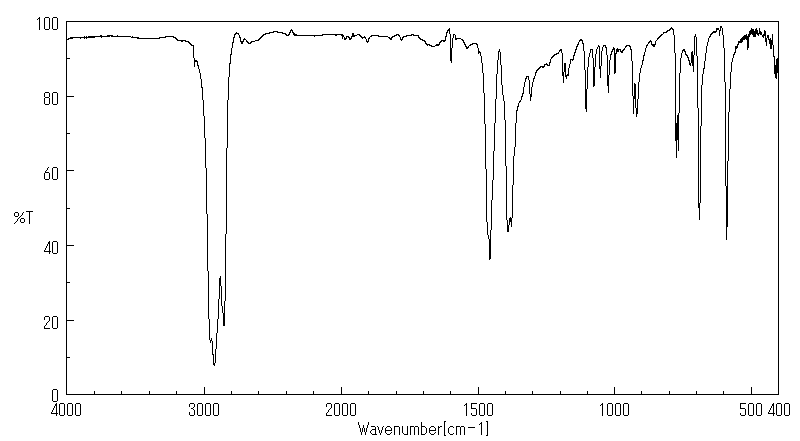
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息