1,2,3,3a,4,6alpha-六氢并环戊二烯 | 5549-09-7
中文名称
1,2,3,3a,4,6alpha-六氢并环戊二烯
中文别名
——
英文名称
bicyclo[3.3.0]oct-2-ene
英文别名
cis-bicyclo<3.3.0>-2-octene;cis-bicyclo<3.3.0>oct-2-ene;1,2,3a,4,6a-hexahydro-6-methylpentalene;Bicyclo<3.3.0>-2-octene;bicyclo<3.3.0>oct-2-ene;2-bicyclo<3.3.0>octene;1,2,3,3a,4,6a-hexahydropentalene
CAS
5549-09-7
化学式
C8H12
mdl
——
分子量
108.183
InChiKey
KEHFJHPSOFFXBO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
保留指数:850
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:8
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
反应信息
-
作为反应物:描述:1,2,3,3a,4,6alpha-六氢并环戊二烯 在 正丁基锂 、 Celite 、 二乙胺 、 间氯过氧苯甲酸 、 pyridinium chlorochromate 作用下, 以 乙醚 、 正己烷 、 二氯甲烷 、 氯仿 为溶剂, 反应 62.0h, 生成 (+/-)-1α,5α-bicyclo[3.3.0]oct-3-en-2-one参考文献:名称:无偏环烯丙基醇的Mitsunobu反应。摘要:在三种不同的溶剂中研究了使用三苯基膦,偶氮二羧酸二乙酯和苯甲酸对无偏烯丙基醇进行立体化学转化(通常称为Mitsunobu反应),并特别注意了产品组成。(R)-3-氘-2-环己烯-1-醇的光延反应与1-氘-5-甲基-2-环己烯-1-醇,1-氘-甲基的顺式和反式异构体产生的结果5-叔丁基-2-环己烯-1-醇,光学活性的顺式和反式5-异丙基-2-甲基-2-环己烯-1-醇在甲醇中心的转化和保留方面均具有相似的产物分布以及使用THF或苯作为溶剂时,以及顺式和反式S(N)2'型加成反应(CH(2)Cl(2)产生的选择性产物分布较少)。有趣的是 已经发现,起始烯丙基醇的准赤道和准轴向性质似乎不影响该反应的产物分布,也没有影响烯丙基醇中心碳的甲基取代。在所有情况下,这些空间无偏的烯丙醇均检测到大量(8-28%)的非S(N)2型产品。当对空间上不要求的(R)-3-氘-2-环己烯-1-醇进行MitsunobuDOI:10.1021/jo9615155
-
作为产物:描述:1,5-cis,cis-cyclooctadiene 在 bis(1,5-cyclooctadiene)nickel (0) 、 三氟化硼乙醚 作用下, 以 甲苯 为溶剂, 以99.5%的产率得到1,2,3,3a,4,6alpha-六氢并环戊二烯参考文献:名称:Cycloisomerization and [2+2]cyclodimerization of 1,5-cyclooctadiene catalyzed with the Ni(COD)2/BF3·OEt2 system摘要:The catalytic system Ni(COD)(2)/BF3 OEt2 has been studied in conversions of 1,5-cyclooctadiene under argon and ethylene atmosphere. It has been demonstrated that the catalytic system formed under argon exhibits a high effectiveness in cycloisomerization of 1,5-COD surpassing in this characteristic all known nickel complex catalysts (selectivity to bicyclo-[3 3 0]-octene-2 is up to 99 5% at 100% conversion). In the case of ethylene atmosphere the system produces mainly dinners (yield of cyclodimers above 70%) It has been shown that the catalytic system Ni(COD)(2)/BF3 OEt2 has the feature of "a living catalyst" consisting in resuming the initial activity with a new portion of 1,5-COD added when the monomer was fully exhausted The main and side products of the 1,5-COD conversion have been identified with GC-MS and preparative liquid chromatography combined with NMR and IR spectroscopy Based on EPR and IR spectroscopic data a mechanism for the catalytic performance of the Ni(COD)(2)/BF3 OEt2 system in argon or ethylene atmospheres is Suggested. It has been shown that Ni(0) is oxidized by the lewis acid to Ni(I) which is stabilized by substrate molecules in a mononuclear form without involvement of conventional organoelement entities Three sorts of paramagnetic nickel species have been found ionic complexes containing pi-coordinated COD ligands; ionic complexes sigma-bonded to COD: complexes as intimate pairs with BF4- counter tons. A mechanism for the catalytic conversion of 1,5-cyclooctadiene is proposed (C) 2009 Elsevier B.V. All rights reserved.DOI:10.1016/j.molcata.2009.09.017
文献信息
-
Direct in situ synthesis of cationic N-heterocyclic carbene iridium and rhodium complexes from neat ionic liquid: Application in catalytic dehydrogenation of cyclooctadiene作者:Ulrich Hintermair、Thibaut Gutel、Alexandra M.Z. Slawin、David J. Cole-Hamilton、Catherine C. Santini、Yves ChauvinDOI:10.1016/j.jorganchem.2008.04.017日期:2008.7to cationic N-heterocyclic carbene (NHC) complexes of rhodium and iridium from neat dialkyl-imidazolium ionic liquids (ILs) has been found. The method uses complexes bearing basic anionic ligands, [M(COD)(PPh3)X], X = OEt, MeCO2, which react with the inactivated imidazolium cation in the absence of external bases yielding one M-NHC moiety and the free protonated base. This new one-pot synthesis leaving
-
Vinyl Carbocations Generated under Basic Conditions and Their Intramolecular C–H Insertion Reactions作者:Benjamin Wigman、Stasik Popov、Alex L. Bagdasarian、Brian Shao、Tyler R. Benton、Chloé G. Williams、Steven P. Fisher、Vincent Lavallo、K. N. Houk、Hosea M. NelsonDOI:10.1021/jacs.9b02110日期:2019.6.12Here we report the surprising discovery that high-energy vinyl carbocations can be generated under strongly basic conditions, and that they engage in intramolecular sp3 C-H insertion reactions through the catalysis of weakly coordinating anion salts. This approach relies on the unconventional combination of lithium hexamethyldisilazide base and the commercially available catalyst, triphenylmethylium
-
Singlet photosensitization of simple alkenes. Part 2. Photochemical transformation of cyclo-octa-1,5-dienes sensitized by aromatic ester作者:Seizi Goto、Setsuo Takamuku、Hiroshi Sakurai、Yoshihisa Inoue、Tadao HakushiDOI:10.1039/p29800001678日期:——Irradiation of an oxygen-free pentane solution of cis,cis- or cis,trans-cyclo-octa-1,5-diene, (1 cc) or (1 ct), in the presence of methyl benzoate as sensitizer gave the cis,trans-isomer and tricyclo[3.3.0.02,6]octane. However, the tricyclo-octane was not an immediate product from (1 cc), but was formed via(1 ct). Kinetic evidence and energetic considerations indicate a non-vertical singlet sensitization在苯甲酸甲酯作为敏化剂的情况下,辐照顺式,顺式或顺式,反式-环-辛-1,5-二烯(1 cc)或(1 ct)的无氧戊烷溶液,得到顺式,反式异构体和三环[3.3.0.0 2,6 ]辛烷。但是,三环辛烷不是(1 cc)的直接产物,而是通过(1 ct)。动力学证据和精力充沛的考虑表明,涉及单线态激基复合物的非垂直单线态敏化机理,其继而分解而留下了环辛二烯的扭曲,激发的单线态。两个激发络合物,实施例1和实施例2的,因此两个扭绞单环-辛二烯,Ç,1个p和吨,1个p,被假定以理顺的(1不同的反应性毫升)和(1克拉)。从淬灭速率常数中未发现两个双键之间跨环相互作用的证据。环辛二烯和环辛烯的猝灭速率常数与氧化电位之间的相关性表明了该激基复合物的电荷转移特性。手性芳族酯敏化的不对称顺式-反式光致异构化也支持具有相当刚性结构的激基复合物的参与。
-
Selective Isomerization of 1,5-Cyclooctadiene to 1,4-Cyclooctadiene Catalyzed by Bis(acetylacetonato)nickel–Triethyldialuminum Trichloride–Phosphorus Ligand作者:Yasumasa Sakakibara、Isao Yamamoto、Ken Sasaki、Mutsuji Sakai、Norito UchinoDOI:10.1246/bcsj.64.2236日期:1991.7The isomerization of 1,5-cyclooctadiene (1,5-COD) with Ni(acac)2–Et3Al2Cl3–phosphorus ligand (Ni : Al2 : P=1 : 10 : 3) was first examined with varying P ligands in toluene in order to find suitable conditions for the formation of 1,4-COD. The product distribution depended largely on the P ligands. For several phosphites possessing very strong π-acceptor properties, the main product was 1,4-COD. In particular, the less-bulky bicyclic phosphite, 4-ethyl-2,6,7-trioxa-1-phosphabicyclo[2.2.2]octane (L-3) was very effective and gave 1,4-COD in a high selectivity of 93% at 66% conversion (1,5-COD/ Ni=500, molar ratio) by employing a low temperature of −30°C. However, the reaction stopped before reaching completion because the catalyst was deactivated by the accumulation of 1,4-COD product. The conversion, depending on the 1,5-COD/toluene ratio (volume) rather than the 1,5-COD/Ni ratio, increased with a decrease in the 1,5-COD/ toluene ratio and was 66–69% at a ratio of 0.19. On the other hand, the catalyst (P: L-3) was much less active for 1,4-COD than for 1,5-COD and was deactivated quickly under the same reaction conditions. This appeared to result in a high selectivity of 1,4-COD in the isomerization of 1,5-COD. The mechanistic implications of the experimental results are discussed.1,5-环辛二烯(1,5-COD)在Ni(acac)2–Et3Al2Cl3–磷配体(Ni : Al2 : P=1 : 10 : 3)催化下的异构化反应,首先在甲苯中对不同的磷配体进行了研究,以寻找适合形成1,4-COD的条件。产物分布在很大程度上依赖于磷配体。对于几种具有非常强的π-受体特性的磷酸酯,主要产物为1,4-COD。尤其是体积较小的双环磷酸酯,4-乙基-2,6,7-三氧-1-磷双环[2.2.2]辛烷(L-3)非常有效,在−30°C的低温下,以66%的转化率在1,5-COD/Ni=500的摩尔比下,获得了93%的1,4-COD高选择性。然而,在达到完全转化前反应停止,因为催化剂因1,4-COD产物的积累而失活。转化率与1,5-COD/甲苯的体积比(而非1,5-COD/Ni的比例)相关,并随着1,5-COD/甲苯比的减少而增加,在比率为0.19时达到了66%-69%。另一方面,催化剂(P: L-3)对1,4-COD的活性远低于对1,5-COD,且在相同的反应条件下迅速失活。这似乎导致1,5-COD异构化中1,4-COD的高选择性。讨论了实验结果的机理意义。
-
Ni0-induzierte CC-verknüpfungen von phenylisocyanat mit cyclischen, fünfgliedrigen alkenen, katalytische herstellung von β,γ-ungesättigten carbonsäureaniliden作者:Heinz Hoberg、Matthias NohlenDOI:10.1016/0022-328x(91)86057-w日期:1991.7Cycloaklenes with a five-membered cyclic system like bicyclo[3.3.0]octene-2- (1a, 2,3-dihydrofuran (1b) and 2,5-dihydrofuran (1c) react with phenylisocyanate on a (Lig)Ni0-system (Lig = tricyclohexylphosphane) to form tricyclohexylphosphane-5-azanickelacylopentan-4-one derivatives by oxidative 1:1 CC-coupling. It has been shown, that these complexes are intermediates of catalytic reactions in which
表征谱图
-
氢谱1HNMR
-
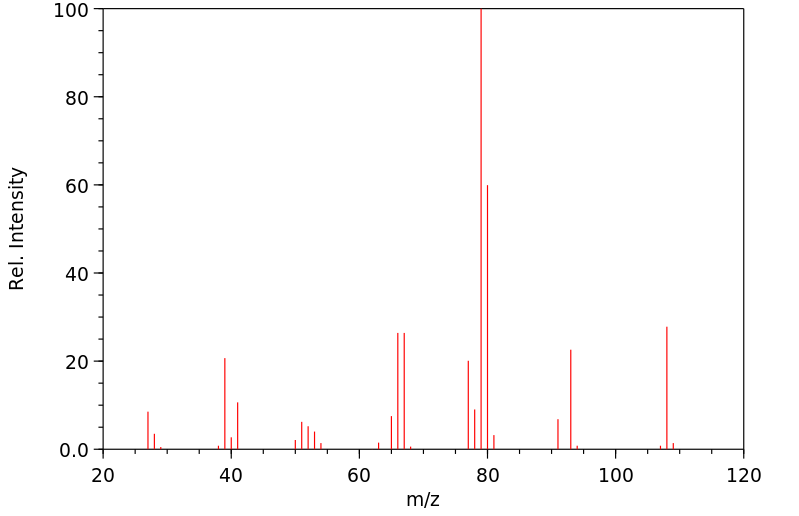
质谱MS
-
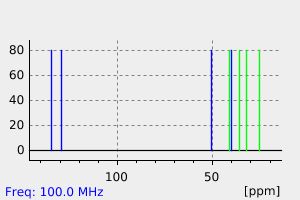
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
降冰片烯
金刚烷-D16
金刚烷
螺戊烷
螺二環己烷
螺[5.6]十二烷
螺[5.5]十一碳-4-烯
螺[5.2]辛-2-烯
螺[4.5]癸烷
螺[4.4]壬-8-烯
螺[3.4]辛烷
螺[3.4]辛-7-烯
螺[3.3]庚-2,5-二烯
螺[2.5]辛烷
螺[2.5]辛-7-烯
螺[2.5]辛-5,7-二烯
螺[2.4]庚-4,6-二烯
螺[2.4]庚-1-烯
螺[2.3]己-1-烯
螺[2.2]戊-1-烯
螺<二环<2.2.2>辛-5-烯-2,1'-环丙烷>
螺<4.4>壬-1,3,7-三烯
螺<4.4>壬-1,3,6,8-四烯
螺(4.4)壬烷
螺(4.4)壬-1,3-二烯
螺(3.4)辛-5,7-二烯
trans-perhydroazulene
萘烷
萘,1,2,3,4,4a,8a-六氢-,顺-
美罗培南中间体F9
篮烷
立方烷
氨基甲硫酸,二甲基-,O,O-(3,3-二甲基1,1-联苯基-2,2-二基)酯
棱晶烷
杜瓦苯
新戊基-1金刚烷
抗氧化剂TH-CPL
庚搭烯
四螺[2.0.2:0.2:0.2:0]十二烷
四螺[2.0.0.0.2.1.1.1]十一烷,顺-
四环己基铅
四环[5.3.0.0<2,6>.0<3,10>]癸-4,8-二烯
四环[5.3.0.0(2,6).0(3,10)]癸烷
四环[4.4.0.02,10.03,7]癸-4,8-二烯
四环[4.2.2.26,5.01,6]十二烷
四环[4.2.0.02,5.03,8]辛烷
四环[3.3.0.02,4.03,6]辛-7-烯
四环<5.3.1.02,6.04,9>十一烷
四环(8.2.2.22,5.26,9)十八碳-1,5,9-三烯
四环(4.1.0.0(2,4).0(3,5))庚烷