1-(3,5-二甲基-1H-吡咯-2-基)-1-乙酮 | 1500-93-2
中文名称
1-(3,5-二甲基-1H-吡咯-2-基)-1-乙酮
中文别名
1-(3,5-二甲基-1H-吡咯基)乙基-1-酮
英文名称
1-(3,5-dimethyl-1H-pyrrol-2-yl)ethan-1-one
英文别名
2-Acetyl-3,5-dimethyl-pyrrol;2,4-Dimethyl-5-acetyl-pyrrol;1-(3,5-dimethyl-1H-pyrrol-2-yl)ethanone
CAS
1500-93-2
化学式
C8H11NO
mdl
MFCD00982312
分子量
137.181
InChiKey
KSZNMNSPWOIFPE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:118 °C
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.375
-
拓扑面积:32.9
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2933990090
-
安全说明:S24/25
SDS
| Name: | 1-(3 5-Dimethyl-1h-pyrrol-2-yl)ethan-1-one tech Material Safety Data Sheet |
| Synonym: | 2-Acetyl-3,5-dimethylpyrrol |
| CAS: | 1500-93-2 |
Synonym:2-Acetyl-3,5-dimethylpyrrol
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1500-93-2 | 1-(3,5-Dimethyl-1H-pyrrol-2-yl)ethan-1 | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1500-93-2: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: brown
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 115 - 117 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H11NO
Molecular Weight: 137
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Bases, strong oxidizing agents, amines.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide, acrid smoke and fumes.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1500-93-2 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1-(3,5-Dimethyl-1H-pyrrol-2-yl)ethan-1-one - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 1500-93-2: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 1500-93-2 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1500-93-2 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 5-乙酰基-2,4-二甲基-1H-吡咯-3-羧酸 5-acetyl-2,4-dimethyl-pyrrole-3-carboxylic acid 17106-15-9 C9H11NO3 181.191 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (E)-1-(3,5-dimethyl-1H-pyrrol-2-yl)-3-pyridin-3-yl-propenone 1171044-03-3 C14H14N2O 226.278
反应信息
-
作为反应物:描述:1-(3,5-二甲基-1H-吡咯-2-基)-1-乙酮 在 盐酸 、 乙醚 、 sodium nitrite 作用下, 生成 1-(4-hydroxyimino-3,5-dimethyl-4H-pyrrol-2-yl)-ethanone参考文献:名称:Kuester, Hoppe-Seyler's Zeitschrift fur Physiologische Chemie, 1922, vol. 121, p. 146摘要:DOI:
-
作为产物:参考文献:名称:Colacicchi, Atti della Accademia Nazionale dei Lincei, Classe di Scienze Fisiche, Matematiche e Naturali, Rendiconti, 1912, vol. <5>21, p. I 657摘要:DOI:
文献信息
-
Copper‐Catalyzed Cope‐Type Hydroamination of Nonactivated Olefins toward Cyclic Nitrones: Scope, Mechanism, and Enantioselective Process Development作者:Mengru Zhang、Shuang Liu、Hexin Li、Yajing Guo、Na Li、Meihui Guan、Haroon Mehfooz、Jinbo Zhao、Qian ZhangDOI:10.1002/chem.201902683日期:2019.9.25remains underdeveloped. Herein we report the copper-catalyzed Cope-type hydroamination of oximes with pendant nonactivated olefins, which enables facile access to a series of five- and six-membered cyclic nitrones under mild conditions. In this study, heterocycle-tethered oximes were employed in the Cope-type hydroamination reaction for the first time. High enantioselectivity was achieved for carbon-tethered
-
Inhibitors of cyclin dependent kinases as anti-cancer agent申请人:——公开号:US20040132746A1公开(公告)日:2004-07-08The present invention relates to 2-substituted 4-heteroaryl-pyrimidines, their preparation, pharmaceutical compositions containing them and their use as inhibitors of cyclin-dependent kinases (CDKs) and hence their use in the treatment of proliferative disorders such as cancer, leukaemia, psoriasis and the like.
-
Organic compounds申请人:Adcock Claire公开号:US20090215776A1公开(公告)日:2009-08-27Compounds of formula I in free or salt or solvate form, where T 1 , T 2 , X, R a , R b , R 8 and R 9 have the meanings as indicated in the specification, are useful for treating inflammatory or obstructive airways, pulmonary hypertension, pulmonary fibrosis, liver fibrosis, muscle diseases and systemic skeletal disorders. Pharmaceutical compositions that contain the compounds and processes for preparing the compounds are also described.式I的化合物以自由形式、盐形式或溶剂合形式存在,其中T1、T2、X、Ra、Rb、R8和R9的含义如规范中所示,可用于治疗炎症或阻塞性气道、肺动脉高压、肺纤维化、肝纤维化、肌肉疾病和全身骨骼疾病。还描述了含有这些化合物的药物组合物和制备这些化合物的方法。
-
Unraveling the Remarkable Influence of Substituents on the Emission Variation and Circularly Polarized Luminescence of Dinuclear Aluminum Triple-Stranded Helicates作者:Kodai Ueno、Yuto Konishi、Luxia Cui、Takunori Harada、Kohei Ishibashi、Takeru Konta、Atsuya Muranaka、Yoshio Hisaeda、Yu Hoshino、Toshikazu OnoDOI:10.1021/acs.inorgchem.4c00045日期:2024.4.8substituent variations on their structural and optical properties. Key findings revealed that the modification of methyl groups to the pyrrole positions significantly extended the conjugation system, resulting in a red shift in the absorption and emission spectra. Conversely, the modification of methyl groups at the methine positions due to steric hindrances increased the torsion angle of the ligands,本研究探索了使用铝的功能性染料的开发,重点关注铝基双核三链螺旋,并研究了取代基变化对其结构和光学性质的影响。主要发现表明,甲基对吡咯位置的修饰显着扩展了共轭系统,导致吸收和发射光谱发生红移。相反,由于空间位阻,次甲基位置上的甲基基团的修饰增加了配体的扭转角,导致吸收和发射光谱的蓝移。所有配合物的一个共同特征是,在激发态下,三个配体之一经历了显着的结构松弛。这导致了明显的斯托克斯位移和最小的光谱重叠与高光致发光行为。此外,我们的研究通过分析所得对映体的手性光学性质,包括其圆二色性和圆偏振发光,扩展到新合成的配合物的光学分辨率。这些见解为新型铝基功能染料的设计和应用提供了宝贵的贡献,可能影响从材料科学到光电子学的一系列领域。
-
Treibs; Fritz, Justus Liebigs Annalen der Chemie, 1958, vol. 611, p. 162,188作者:Treibs、FritzDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
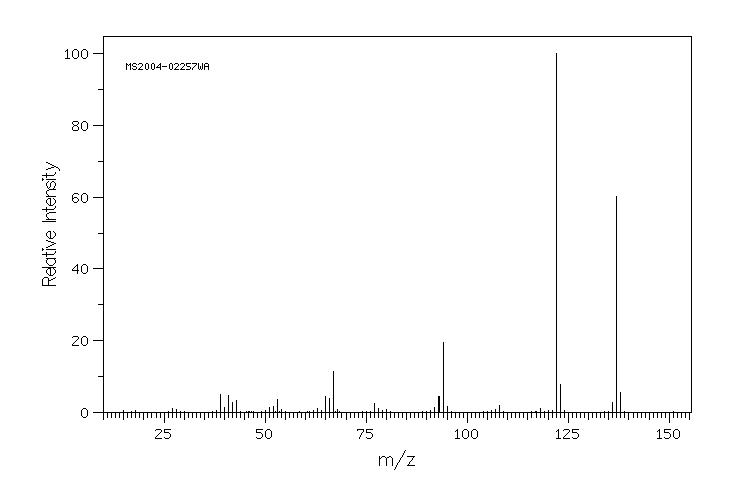
质谱MS
-
碳谱13CNMR
-
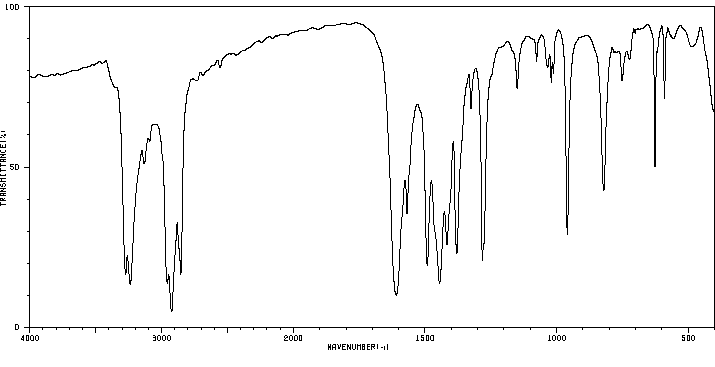
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷