2-甲基戊酰氯 | 5238-27-7
中文名称
2-甲基戊酰氯
中文别名
——
英文名称
2-methylvaleryl chloride
英文别名
2-methylpentanoyl chloride;2-methyl pentanoic acid chloride
CAS
5238-27-7
化学式
C6H11ClO
mdl
MFCD00013656
分子量
134.606
InChiKey
MFIQXAVMTLKUJR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:140-144 °C(lit.)
-
密度:0.978 g/mL at 25 °C(lit.)
-
闪点:100 °F
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:8
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.833
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险等级:8
-
危险品标志:C
-
安全说明:S26,S27,S36/37/39,S45
-
危险类别码:R10,R34
-
WGK Germany:3
-
危险品运输编号:UN 2920 8
-
海关编码:2915900090
-
包装等级:II
-
危险类别:8
-
储存条件:请将药品存放在避光、阴凉干燥处,并密封保存。
SDS
| Name: | 2-Methylvaleryl chloride 98% Material Safety Data Sheet |
| Synonym: | 2-Methylpentanoyl chlorid |
| CAS: | 5238-27-7 |
Synonym:2-Methylpentanoyl chlorid
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 5238-27-7 | 2-Methylvaleryl chloride | 98% | 226-035-3 |
Risk Phrases: 10 34
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Flammable. Causes burns.
Potential Health Effects
Eye:
Causes eye burns.
Skin:
Causes skin burns.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. Causes gastrointestinal tract burns.
Inhalation:
Causes chemical burns to the respiratory tract.
Chronic:
Chronic exposure may cause effects similar to those of acute exposure.
Section 4 - FIRST AID MEASURES
Eyes: Get medical aid immediately. Do NOT allow victim to rub eyes or keep eyes closed. Extensive irrigation with water is required (at least 30 minutes).
Skin:
Get medical aid immediately. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid immediately. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If breathing is difficult, give oxygen. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Vapors can travel to a source of ignition and flash back. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Flammable liquid and vapor. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Use water spray to cool fire-exposed containers. Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Use a spark-proof tool. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Ground and bond containers when transferring material. Use spark-proof tools and explosion proof equipment. Do not breathe dust, vapor, mist, or gas. Do not get in eyes, on skin, or on clothing.
Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep container tightly closed. Do not ingest or inhale. Use only in a chemical fume hood. Discard contaminated shoes.
Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames. Keep away from heat, sparks and flame.
Storage:
Keep away from sources of ignition. Store in a cool, dry place.
Store in a tightly closed container. Flammables-area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use only under a chemical fume hood. Use adequate general or local explosion-proof ventilation to keep airborne levels to acceptable levels.
Exposure Limits CAS# 5238-27-7: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: colorless
Odor: stench
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 140 - 144 deg C
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: 37 deg C ( 98.60 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 0.978
Molecular Formula: C6H11ClO
Molecular Weight: 134.5294
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not currently available.
Conditions to Avoid:
Ignition sources, excess heat.
Incompatibilities with Other Materials:
Strong oxidizing agents, alcohols, strong bases.
Hazardous Decomposition Products:
Hydrogen chloride, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Will not occur.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 5238-27-7 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2-Methylvaleryl chloride - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: Pseudomonas putida:
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: CORROSIVE LIQUID, FLAMMABLE, N.O.S.*
Hazard Class: 8
UN Number: 2920
Packing Group: II
IMO
Shipping Name: CORROSIVE LIQUID, FLAMMABLE, N.O.S.
Hazard Class: 8
UN Number: 2920
Packing Group: II
RID/ADR
Shipping Name: CORROSIVE LIQUID, FLAMMABLE, N.O.S.
Hazard Class:
UN Number: 2920
Packing group: II
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: C
Risk Phrases:
R 10 Flammable.
R 34 Causes burns.
Safety Phrases:
S 9 Keep container in a well-ventilated place.
S 16 Keep away from sources of ignition - No
smoking.
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 33 Take precautionary measures against static
discharges.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 5238-27-7: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 5238-27-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 5238-27-7 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:参考文献:名称:Preparation of Aliphatic Ketones from Lithium Alkyls and Dimethylamides摘要:DOI:10.1021/jo01087a605
-
作为产物:描述:参考文献:名称:Hommelen, Bulletin des Societes Chimiques Belges, 1933, vol. 42, p. 243,249摘要:DOI:
文献信息
-
Antinociceptive effect of extracts and compounds from Hofmeisteria schaffneri作者:Guadalupe Angeles-López、Araceli Pérez-Vásquez、Francisco Hernández-Luis、Myrna Déciga-Campos、Robert Bye、Edelmira Linares、Rachel MataDOI:10.1016/j.jep.2010.07.009日期:2010.9exposure for short space of time. On the other hand, the extracts showed significant antinociceptive effect when tested in the hot plate model. The most active natural product as antinociceptive agent was hofmeisterin III (1) which also was the most stable in the stability study. Its pharmacological effect seems to be partially mediated by an opioid mechanism since naloxone inhibits its action. Using compound民族药理学相关性Hofmeisteria schaffneri(菊科)是一种药用植物,在墨西哥城最重要的市场中被广泛商业化,用于治疗胃肠道不适和皮肤病。研究目的本研究的主要目标是在动物模型中建立沙夫(Hofmeisteria schaffneri)的几种制剂和化合物的潜在急性毒性和抗伤害感受活性。材料与方法分别通过注入,浸渍和加氢蒸馏来制备沙夫霍夫甲的水和有机提取物以及香精油。急性毒性的研究通过Lorke方法完成。使用扭体试验和热板试验评估抗伤害感受作用。通过标准植物化学方法分离天然化合物。另外,通过化学合成制备了一些百里酚酯。通过测量其在37摄氏度下对猪肝硬脂酸酶和小鼠血浆的水解敏感性,定性分析了天然酯和合成酯的稳定性。结果每种测试制剂的LD(50)均高于5000 mg / kg,表明它们没有短时间暴露后对小鼠有毒。另一方面,当在热板模型中进行测试时,提取物显示出明显的镇痛作用。作为抗伤
-
Studies on hypolipidemic agents. II Synthesis of 1-arenesulfonyloxy-2-alkanone derivatives as potent esterase inhibitors and hypolipidemic agents.作者:KAZUO OGAWA、TADAFUMI TERADA、YOSHIYUKI MURANAKA、TOSHIHIRO HAMAKAWA、SADAO HASHIMOTO、SETSURO FUJIIDOI:10.1248/cpb.34.3252日期:——Many 2-oxoalkyl arenesulfonate derivatives having straight or branched alkyl chains of different lengths, 2-oxoalkyl bis-arenesulfonate derivatives, and alkyl arenesulfonate derivatives having a ketal moiety at the 2-position on the alkyl chain were synthesized, and their esterase-inhibitory activities, as well as hypolipidemic activities, were evaluated.Among these compounds, 1-(2, 4, 6-trimethylbenzenesulfonyloxy)-2-dodecanone (III-1u), and 1-(2, 3, 4, 6-tetramethylbenzenesulfonyloxy)-2-hexanone (III-1w), -2-octanone (III-1x) and -2-decanone (III-1y) exhibited potent esterase-inhibitory activities (IC50=3×10-10, 2×10-10, 2×10<-10> and 3×<-11>M, respectively). However, the sulfonate (XV) having a ketal moiety on the alkyl chain and the bis-sulfonate (XVI) exhibited low inhibitory activities toward esterase in comparison with III and XII. Most of the compounds III and some of the compounds XII exhibited potent hypolipidemic activities corresponding to more than 50% lipid-lowering effect (plasma triglyceride and cholesterol ester) in vivo. The structure-activity relatioinships of these compounds are discussed.合成了许多具有直链或支链不同长度烷基链的2-氧代烷基芳磺酸盐衍生物、2-氧代烷基双芳磺酸盐衍生物以及在烷基链的2-位具有缩酮部分的烷基芳磺酸盐衍生物,并评估了它们的酯酶抑制活性及降血脂活性。在这些化合物中,1-(2,4,6-三甲基苯磺酰氧基)-2-十二烷酮(III-1u)、1-(2,3,4,6-四甲基苯磺酰氧基)-2-己烷酮(III-1w)、-2-辛烷酮(III-1x)和-2-癸烷酮(III-1y)表现出强效的酯酶抑制活性(IC50分别为3×10-10、2×10-10、2×10-10和3×10-11M)。然而,相对于III和XII,具有烷基链上缩酮部分的磺酸盐(XV)和双磺酸盐(XVI)对酯酶的抑制活性较低。大多数化合物III和部分化合物XII表现出强效的降血脂活性,对应于体内超过50%的脂质降低效果(血浆甘油三酯和胆固醇酯)。讨论了这些化合物的构效关系。
-
ETHYNYL DERIVATIVES申请人:Hoffmann-La Roche Inc.公开号:US20130090347A1公开(公告)日:2013-04-11The present invention relates to ethynyl derivatives of formula I wherein U, V, W, Y, R, R 1 , R 2 , R 3 and R 3′ are as described herein. It has been found that the compounds of general formula I are allosteric modulators of the metabotropic glutamate receptor subtype 5 (mGluR5).
-
Phenylglycine Methyl Ester, a Useful Tool for Absolute Configuration Determination of Various Chiral Carboxylic Acids作者:Tetsuya Yabuuchi、Takenori KusumiDOI:10.1021/jo991218a日期:2000.1.1A new chiral anisotropic reagent, phenylglycine methyl ester (PGME), developed for the elucidation of the absolute configuration of chiral alpha,alpha-disubstituted acetic acids, has turned out to be applicable to other substituted carboxylic acids, such as chiral alpha-hydroxy-, alpha-alkoxy-, and alpha-acyloxy-alpha, alpha-disubstituted acetic acids, as well as to chiral beta, beta-disubstituted
-
Esters of 3-hydroxyindone compounds as herbicides and miticides申请人:Union Carbide Corporation公开号:US04104043A1公开(公告)日:1978-08-01A new series of substituted esters of 3-hydroxyindone compounds have been found to have exceptional miticidal and herbicidal activity.一系列新的3-羟基吲哚化合物的取代酯被发现具有出色的杀螨和除草活性。
表征谱图
-
氢谱1HNMR
-
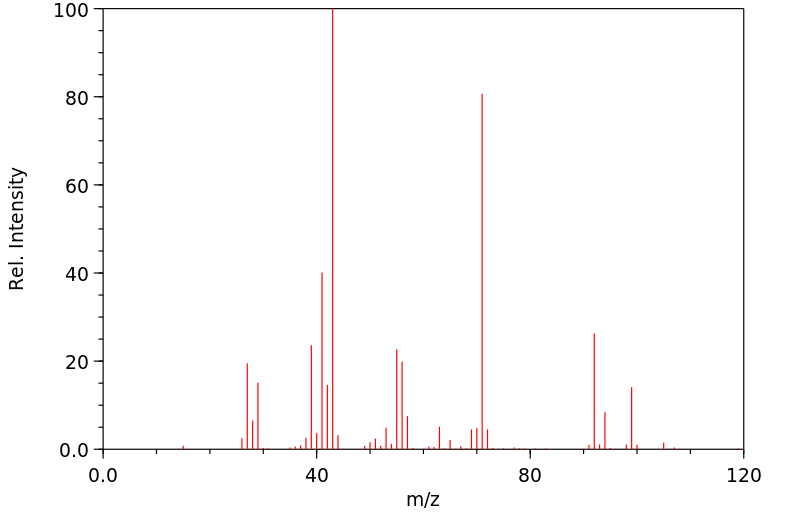
质谱MS
-
碳谱13CNMR
-
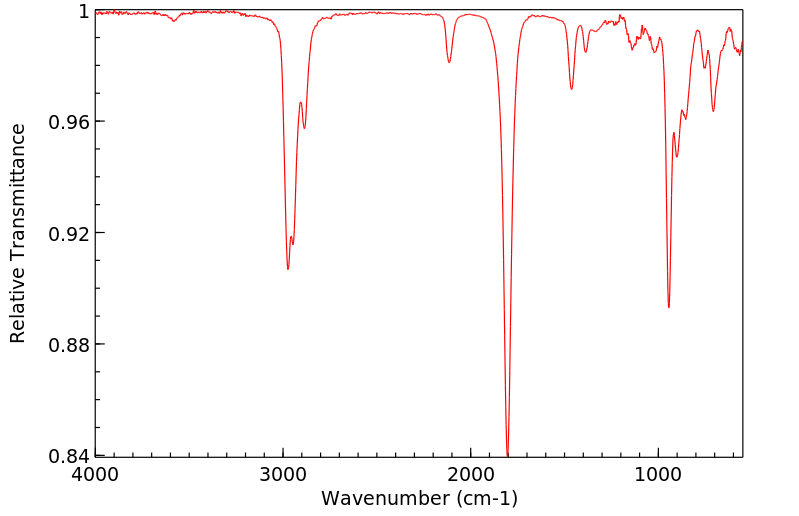
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-六氢-3a(1H)-并环戊二烯羰基氯化物
金刚烷酰氯
辛酰溴
辛酰氯
辛酰氟
辛-5t-烯酰氯
衣康酰氯
螺[3.5]壬烷-2-甲酰氯
螺[3.4]辛烷-2-甲酰氯
草酰溴
草酰氯
草酰氟
花生酰氯
花生四烯酰氯
肉豆蔻酰氯
肉豆蔻酰-1-13C氯
癸酰氯
癸基二酰二氯
癸二酰氯
異丁醯溴
甲酰氯
甲酰基溴化物
甲氧基乙酰氯
甲基丙烯酰氯
甲基丙烯酰氟
环辛烷羰基氯化物
环戊烷羰基溴
环戊烷羰基氟
环戊基甲酰氯
环戊基乙酰氯
环戊基乙酰氯
环庚烷羰酰氯
环己酰溴
环己甲酰氯
环己基乙酰氯
环己-3-烯-1-甲酰氯
环丙烷乙酰氯
环丙烷丙酰氯
环丁烷羰基碘化物
环丁烷羰基溴化物
环丁烷-1,2-二甲酰氯
环丁基甲酰氯
溴二氯乙酰氯
溴乙酰溴
溴乙酰氯
溴(二氟)乙酰氯
油酰氯
氰基乙酰溴
氯碘乙酰氯
氯氟乙酰氟